|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C4H3FN2O2 |
||||||||||
| Molecular Weight | 130.08 | CAS No. | 51-21-8 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 26 mg/mL (199.87 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | 5-FU (5-Fluorouracil) is a DNA/RNA synthesis inhibitor, which interrupts nucleotide synthetic by inhibiting thymidylate synthase (TS) in tumor cells. Fluorouracil induces apoptosis and can be used in the treatment of HIV. | |
|---|---|---|
| Targets |
|
|
| In vitro | Adrucil is an analogue of uracil with a fluorine atom at the C-5 position in place of hydrogen. It rapidly enters the cell using the same facilitated transport mechanism as uracil. Adrucil is converted intracellularly to several active metabolites: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP). The Adrucil metabolite FdUMP binds to the nucleotide-binding site of TS, forming a stable ternary complex with the enzyme and CH2THF, thereby blocking binding of the normal substrate dUMP and inhibiting dTMP synthesis. Metabolite of Adrucil also can be misincorporated into DNA, leading to DNA strand breaks and cell death. The pro-apoptosis effects of Adrucil may be related to its activation of tumor suppressor p53. Loss of p53 function reduces cellular sensitivity to Adrucil. [1] Adrucil is able to inhibit the survival and induce apoptosis of a board range of cancer cells. Adrucil suppresses viabilities of the nasopharyngeal carcinoma cell line CNE2 and HONE1 [2], pancreatic cancer cell lines Capan-1 [3], and human colon carcinoma cell line HT-29 [4] with IC50 of 9 μg/mL, 3 μg/mL, 0.22 μM, 2.5 μM, respectively. | |
| In vivo | Adrucil is widely used in the treatment of a range of cancers, including colorectal and breast cancers. [1] 100mg/kg Adrucil significantly suppresses tumor growth of murine colon carcinomas Colon 38 with tumor-doubling time (TD), growth-delay factor (GDF), and T/C of 26.5 days, 4.4, and 14%. [5] |
Protocol (from reference)
| Cell Assay:[4] |
|
|---|---|
| Animal Study:[5] |
|
References
Customer Product Validation
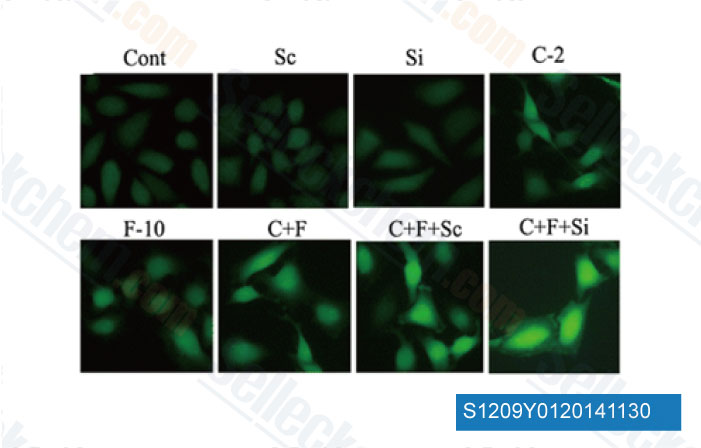
-
Data from [Data independently produced by Mol Cell Biochem, 2014, 10.1007/s11010-014-2253-6]

-
Data from [Data independently produced by , , J Invest Dermatol, 2018, 138(8):1716-1725]
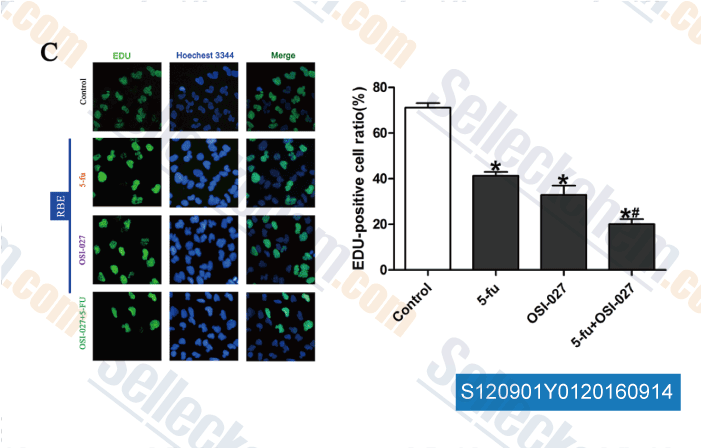
-
Data from [Data independently produced by , , Eur Rev Med Pharmacol Sci, 2016, 20(9):1699-706.]
Selleck's 5-FU (5-Fluorouracil) has been cited by 238 publications
| Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer [ Cell Death Dis, 2024, 15(3):187] | PubMed: 38443386 |
| A functional personalised oncology approach against metastatic colorectal cancer in matched patient derived organoids [ NPJ Precis Oncol, 2024, 8(1):52] | PubMed: 38413740 |
| Novel dual action PARP and microtubule polymerization inhibitor AMXI-5001 powerfully inhibits growth of esophageal carcinoma both alone and in combination with radiotherapy [ Am J Cancer Res, 2024, 14(1):378-389] | PubMed: 38323288 |
| Molecular Mechanisms of Drug Resistance And Strategies of Sensitization in Breast Cancer [ Frontiers, 2024, 10.3389/978-2-8325-4184-5] | PubMed: none |
| ST6GAL1 is associated with poor response to chemoradiation in rectal cancer [ Neoplasia, 2024, 51:100984] | PubMed: 38467087 |
| The ribosomal protein L22 binds the MDM4 pre-mRNA and promotes exon skipping to activate p53 upon nucleolar stress [ bioRxiv, 2024, 10.1101/2023.12.29.573614] | PubMed: none |
| The ribosomal protein L22 binds the MDM4 pre-mRNA and promotes exon skipping to activate p53 upon nucleolar stress [ bioRxiv, 2024, 10.1101/2023.12.29.573614] | PubMed: none |
| Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer [ Nat Med, 2023, 29(3):605-614] | PubMed: 36864254 |
| Lysine butyrylation of HSP90 regulated by KAT8 and HDAC11 confers chemoresistance [ Cell Discov, 2023, 9(1):74] | PubMed: 37460462 |
| Colorectal Cancer Organoid-Stroma Biobank Allows Subtype-Specific Assessment of Individualized Therapy Responses [ Cancer Discov, 2023, 13(10):2192-2211] | PubMed: 37489084 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
