|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C35H46FN5O9S |
|||
| Molecular Weight | 731.83 | CAS No. | 850876-88-9 | |
| Solubility (25°C)* | In vitro | DMSO | 144 mg/mL (196.76 mM) | |
| Ethanol | 144 mg/mL (196.76 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Danoprevir is a peptidomimetic inhibitor of the NS3/4A protease of hepatitis C virus (HCV) with IC50 of 0.2-3.5 nM, inhibition effect for HCV genotypes 1A/1B/4/5/6 is ~10-fold higher than 2B/3A. Phase 2. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Danoprevir (0.29 nM) inhibits the reference genotype 1 NS3/4A protease half-maximally, but a high dose of Danoprevir (10 μM) shows no appreciably inhibition in a panel of 79 proteases, ion channels, transporters, and cell surface receptors. Danoprevir remains bound to and inhibits NS3/4A for more than 5 hours after its initial association. Danoprevir (45 nM) eliminates a patient-derived HCV genotype 1b replicon from hepatocyte-derived Huh7 cells with an EC50 of 1.8 nM. [1] In HCV subgenomic replicon cell lines containing the individual mutations, V36M, R109K, and V170A substitutions confer little or no resistance to Danoprevir, but the R155K substitution confers a high level (62-fold increase) of resistance to Danoprevir. [2] In Huh7.5 cells transfected with chimeric recombinant virus, Danoprevir shows antiviral inhibition effects against HCV genotypes 1, 4 and 6 with IC50 of 2-3 nM, which are >100-fold lower than genotypes 2/3/5 (280-750 nM). [3] | ||
| In vivo | Danoprevir (30 mg/kg) administered to rats or monkeys shows that its concentrations in liver 12 hours after dosing exceed the Danoprevir concentration required to eliminate replicon RNA from cells. [1] | ||
| Features | A peptidomimetic inhibitor of the NS3/4A protease of hepatitis C virus (HCV). |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[1] |
|
References
|
Customer Product Validation
-
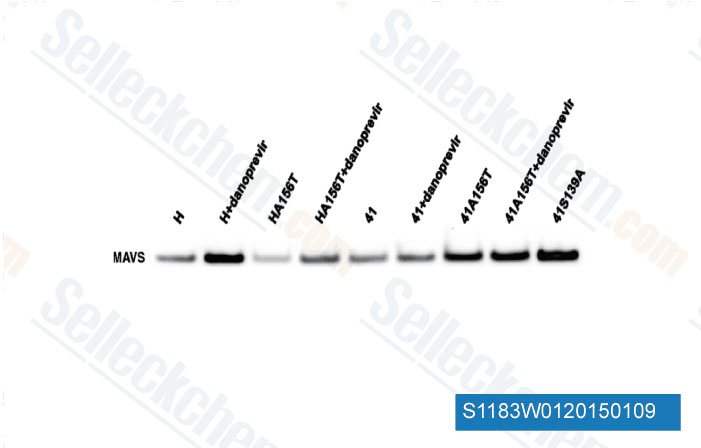
Data from [Data independently produced by Mol Biol Evol, 2014, 31(6), 1546-53]
-
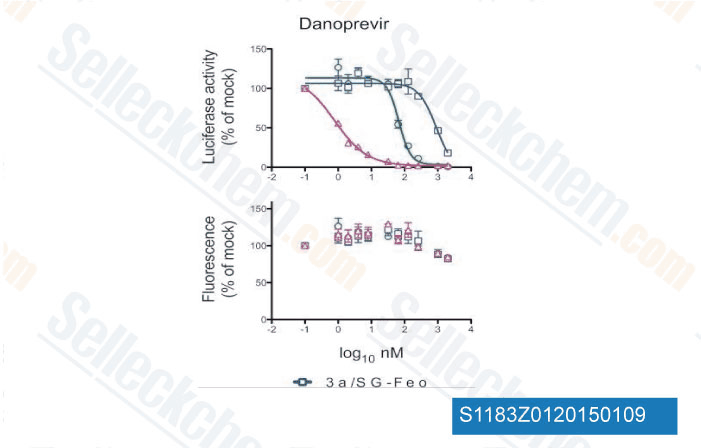
Data from [Data independently produced by Antimicrob Agents Chemother, 2012, 56(10), 5365-73]
-
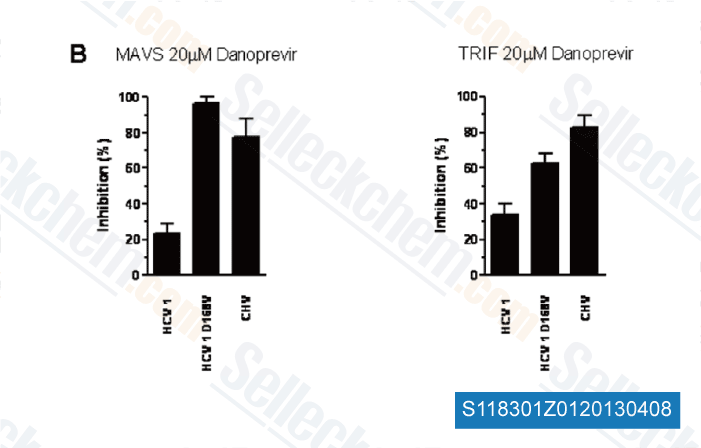
Data from [PLoS One, 2012, 7, e42481]
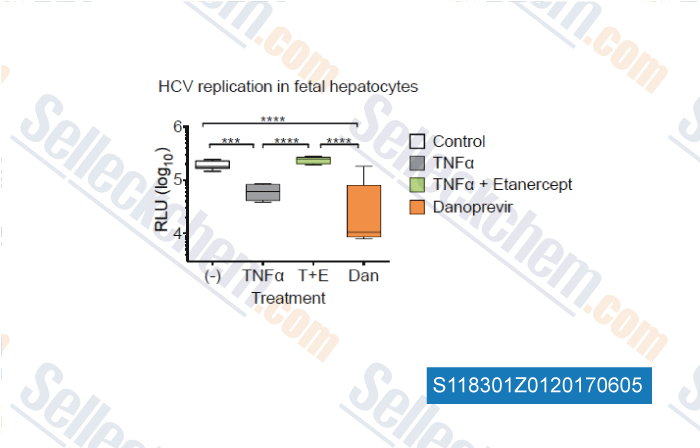
-
, , Gastroenterology, 2017, 153(2):566-578
Selleck's Danoprevir has been cited by 17 publications
| Preclinical characterization of a non-peptidomimetic HIV protease inhibitor with improved metabolic stability [ Antimicrob Agents Chemother, 2024, 68(4):e0137323.] | PubMed: 38380945 |
| Mechanisms of Action of the Host-Targeting Agent Cyclosporin A and Direct-Acting Antiviral Agents against Hepatitis C Virus [ Viruses, 2023, 15(4)981] | PubMed: 37112961 |
| RECOVER identifies synergistic drug combinations in vitro through sequential model optimization [ Cell Rep Methods, 2023, 3(10):100599] | PubMed: 37797618 |
| Integrative multiomics and in silico analysis revealed the role of ARHGEF1 and its screened antagonist in mild and severe COVID-19 patients [ J Cell Biochem, 2022, 10.1002/jcb.30213] | PubMed: 35037717 |
| Hepatitis C virus drugs that inhibit SARS-CoV-2 papain-like protease synergize with remdesivir to suppress viral replication in cell culture [ Cell Rep, 2021, 35(7):109133] | PubMed: 33984267 |
| Characterization of fluorescent probe substrates to develop an efficient high-throughput assay for neonatal hepatic CYP3A7 inhibition screening [ Sci Rep, 2021, 11(1):19443] | PubMed: 34593846 |
| Visualization of Positive and Negative Sense Viral RNA for Probing the Mechanism of Direct-Acting Antivirals against Hepatitis C Virus. [ Viruses, 2019, 11(11)] | PubMed: 31717338 |
| Modular cell-based platform for high throughput identification of compounds that inhibit a viral interferon antagonist of choice [Vasou A, et al. Antiviral Res, 2018, 150:79-92] | PubMed: 29037975 |
| Tumor Necrosis Factor Inhibits Spread of Hepatitis C Virus Among Liver Cells, Independent from Interferons. [Laidlaw SM, et al. Gastroenterology, 2017, 10.1053/j.gastro.2017.04.021] | PubMed: 28456632 |
| Quantifying antiviral activity optimizes drug combinations against hepatitis C virus infection. [ Proc Natl Acad Sci U S A, 2017, 114(8):1922-1927] | PubMed: 28174263 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
