|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C21H25ClO6 |
|||
| Molecular Weight | 408.87 | CAS No. | 461432-26-8 | |
| Solubility (25°C)* | In vitro | DMSO | 82 mg/mL (200.55 mM) | |
| Ethanol | 82 mg/mL (200.55 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Dapagliflozin is a potent and selective hSGLT2 inhibitor with EC50 of 1.1 nM, exhibiting 1200-fold selectivity over hSGLT1. Phase 4. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Dapagliflozin is not sensitive to hSGLT1 with a 1200-fold IC50. [1] Dapagliflozin is 32-fold more potent than phlorizin against hSGLT2 but 4-fold less than phlorizin against hSGLT1. Dapagliflozin is highly selective versus GLUT transporters and displays 8–9% inhibition in protein-free buffer at 20 μM and virtually no inhibition in the presence of 4% bovine serum albumin. [2] Dapagliflozin has good permeability across Caco-2 cell membranes and is a substrate for P-glycoprotein (P-gp) but not a significant P-gp inhibitor. Dapagliflozin is stable in rat, dog, monkey, and human serum at 10 μM. Dapagliflozin shows no inhibitory responses or induction to human P450 enzymes. The in vitro metabolic pathways Dapagliflozin are glucuronidation, hydroxylation, and O-deethylation. [3] |
||
| In vivo | Dapagliflozin reduces blood glucose levels by 55% after 0.1 mg/kg oral dose in hyperglycemic streptozotocin (STZ) rats, which is in part to the metabolic stability conferred by the C-glucoside linkage. Dapagliflozin displays a favorable absorption, distribution, metabolism, and excretion (ADME) profile and is orally bioavailable. [1] Dapagliflozin (1 mg/kg) causes significant dose-dependent glucosuria and increase in urine volume in normal rats over 24 hours post-dose. Dapagliflozin induces increase in urine glucose and urine volume excretion at 6 hours post-dose in Zucker diabetic fatty (ZDF) rats. Dapagliflozin lowers fasting and fed glucose levels in ZDF rats even by 2 weeks of treatment, without any marker of renal or liver toxicity. [2] Dapagliflozin significantly reduces the development of hyperglycaemia, with lowered blood glucose. Dapagliflozin could improve the insulin sensitivity, reduce β-cell mass and the development of impaired pancreatic function. [4] |
||
| Features | More potent stimulator of glucosuria than other SGLT2 inhibitors. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
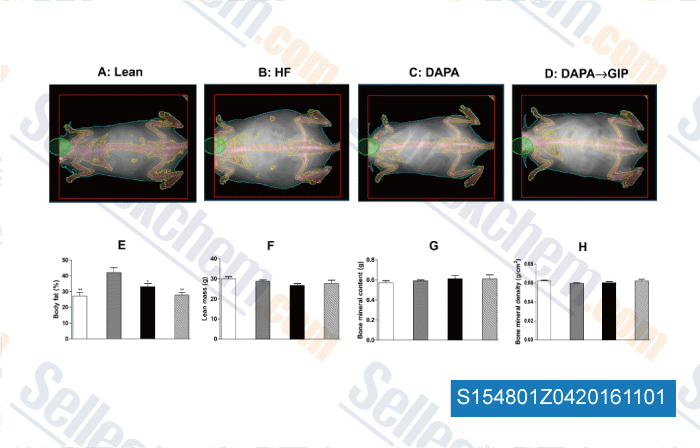
-
, , Mol Cell Endocrinol, 2016, 420:37-45.
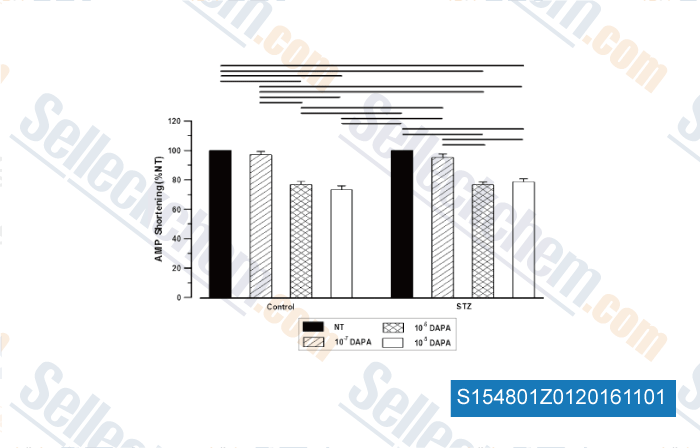
-
, , Mol Cell Biochem,2015, 400(1-2):57-68.

-
Data from [Data independently produced by , , Mol Metab, 2016, 5(10):1048-56.]
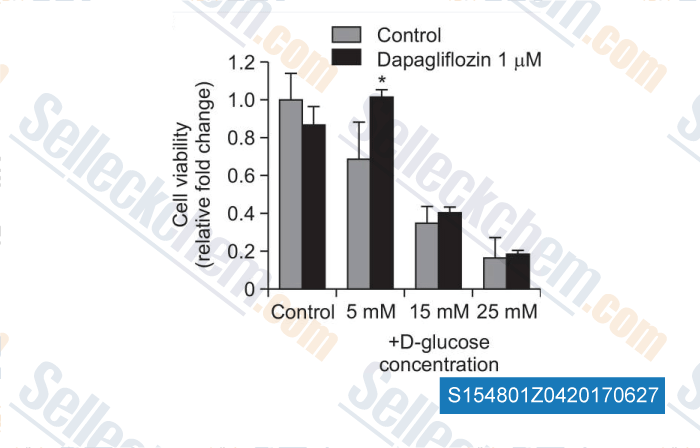
-
Data from [Data independently produced by , , Biomol Ther, 2016, 24(4):363-70]
Selleck's Dapagliflozin has been cited by 45 publications
| Single-nucleus RNA sequencing identifies cell-type-specific effects of sodium-glucose co-transporter 2 inhibitors in human myocardial slices [ Eur Heart J, 2024, 31:ehae472.] | PubMed: 39082743 |
| ALDH2 mediates the effects of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on improving cardiac remodeling [ Cardiovasc Diabetol, 2024, 23(1):380] | PubMed: 39462342 |
| SGLT2i improves kidney senescence by down-regulating the expression of LTBP2 in SAMP8 mice [ J Cell Mol Med, 2024, 28(6):e18176] | PubMed: 38454800 |
| SGLT2i improves kidney senescence by down-regulating the expression of LTBP2 in SAMP8 mice [ J Cell Mol Med, 2024, 28(6):e18176] | PubMed: 38454800 |
| Dapagliflozin improves skeletal muscle insulin sensitivity through SIRT1 activation induced by nutrient deprivation state [ Sci Rep, 2024, 14(1):16878] | PubMed: 39043740 |
| SGLT2 inhibitors attenuate endothelial to mesenchymal transition and cardiac fibroblast activation [ Sci Rep, 2024, 14(1):16459] | PubMed: 39013942 |
| Dapagliflozin Mitigated Elevated Disomic and Diploid Sperm in a Mouse Model of Diabetes and Recover the Disrupted Ogg1, Parp1, and P53 Gene Expression [ Biomedicines, 2023, 10.3390/biomedicines11112980] | PubMed: 38001980 |
| Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice [ Biomedicines, 2023, 11(10)2674] | PubMed: 37893048 |
| Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice [ Biomedicines, 2023, 11(10)2674] | PubMed: 37893048 |
| Streptozotocin induces renal proximal tubular injury through p53 signaling activation [ Sci Rep, 2023, 13(1):8705] | PubMed: 37248327 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
