|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C12H9F3N2O2 |
|||
| Molecular Weight | 270.21 | CAS No. | 75706-12-6 | |
| Solubility (25°C)* | In vitro | DMSO | 54 mg/mL (199.84 mM) | |
| Ethanol | 54 mg/mL (199.84 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Leflunomide is a pyrimidine synthesis and protein tyrosine kinase inhibitor belonging to the DMARD, used as an immunosuppressant agent. The active metabolite of Leflunomide is A77 1726, which inhibits dihydroorotate dehydrogenase (DHODH). Leflunomide is also an agonist of the AhR. | |
|---|---|---|
| Targets |
|
|
| In vitro | Leflunomide is actually a prodrug that is processed in vivo to the active metabolite A771726,which has been shown to inhibit proliferation of mononuclear and T-cells. Leflunomide is an inhibitor of several protein tyrosine kinases, with IC50 values between 30 mM and 100 mM in vitro cellular and enzymatic assays. [1] Leflunomide is capable of inhibiting anti-CD3- and interleukin-2 (IL-2)-stimulated T cell proliferation. Leflunomide is able to inhibit p59fyn and p56lck activity in in vitro tyrosine kinase assays. Leflunomide also inhibits Ca2+ mobilization in Jurkat cells stimulated by anti-CD3 antibody but not in those stimulated by ionomycin. Leflunomide also inhibits distal events of anti-CD3 monoclonal antibody stimulation, namely, IL-2 production and IL-2 receptor expression on human T lymphocytes. Leflunomide also inhibits tyrosine phosphorylation in CTLL-4 cells stimulated by IL-2. [2] Leflunomide is an immunomodulatory drug that may exert its effects by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), which plays a key role in the de novo synthesis of the pyrimidine ribonucleotide uridine monophosphate (rUMP). Leflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with the cell cycle progression due to inadequate production of rUMP and utilizing mechanisms involving p53. [3] | |
| In vivo | Leflunomide is able to prevent and reverse allograft and xenograft rejection in rodents, dogs, and monkeys. [2] |
Protocol (from reference)
References
Customer Product Validation
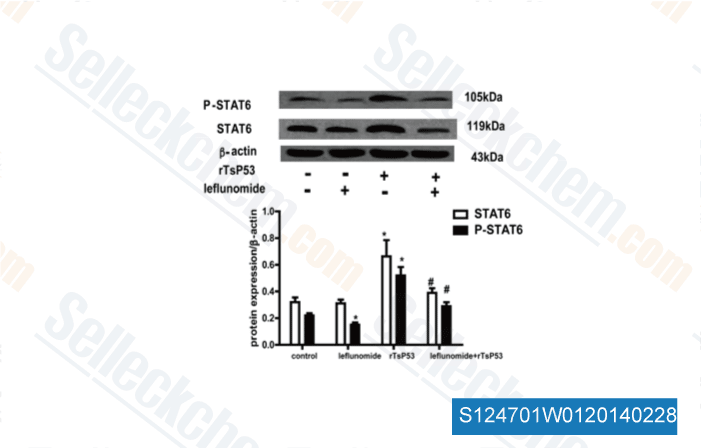
-
Data from [Cell Immunol, 2014, 288(1-2), 1-7]

-
Data from [Cell Immunol, 2014, 288(1-2), 1-7]

-
Data from [Cell Immunol, 2014, 288(1-2), 1-7]
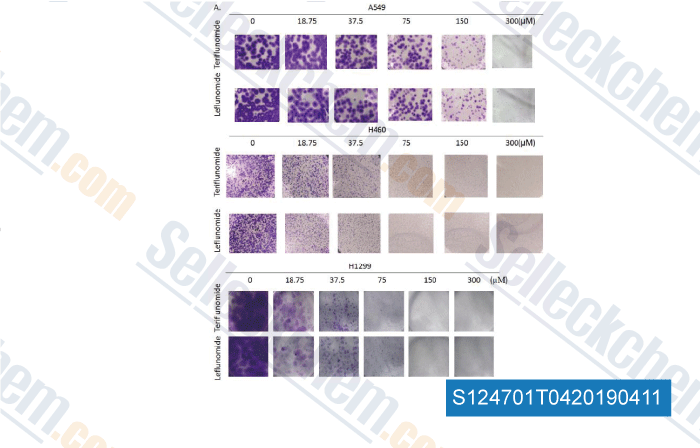
-
Data from [Data independently produced by , , Toxicol Lett, 2018, ]
Selleck's Leflunomide has been cited by 16 publications
| IDH2 stabilizes HIF-1α-induced metabolic reprogramming and promotes chemoresistance in urothelial cancer [ EMBO J, 2023, e110620.] | PubMed: 36637036 |
| Role of mitochondrial fusion proteins MFN2 and OPA1 on lung cellular senescence in chronic obstructive pulmonary disease [ Respir Res, 2023, 24(1):319] | PubMed: 38110986 |
| Dihydroorotate dehydrogenase promotes cell proliferation and suppresses cell death in esophageal squamous cell carcinoma and colorectal carcinoma [ Transl Cancer Res, 2023, 12(9):2294-2307] | PubMed: 37859742 |
| Dihydroorotate dehydrogenase promotes cell proliferation and suppresses cell death in esophageal squamous cell carcinoma and colorectal carcinoma [ Transl Cancer Res, 2023, 12(9):2294-2307] | PubMed: 37859742 |
| De novo pyrimidine synthesis fuels glycolysis and confers chemoresistance in gastric cancer [ Cancer Lett, 2022, S0304-3835(22)00321-4] | PubMed: 35921972 |
| UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism [ Cell Death Dis, 2022, 13(2):154] | PubMed: 35169125 |
| Therapeutic targeting of both dihydroorotate dehydrogenase and nucleoside transport in MYCN-amplified neuroblastoma [ Cell Death Dis, 2021, 12(9):821] | PubMed: 34462431 |
| Exosome-Depleted Excretory-Secretory Products of the Fourth-Stage Larval Angiostrongylus cantonensis Promotes Alternative Activation of Macrophages Through Metabolic Reprogramming by the PI3K-Akt Pathway [ Front Immunol, 2021, 12:685984] | PubMed: 34367145 |
| In vitro evaluation of disease-modifying antirheumatic drugs against rheumatoid arthritis associated pathogens of the oral microflora [ RMD Open, 2021, 7(3)e001737] | PubMed: 34588273 |
| Leflunomide Inhibits rat-to-Mouse Cardiac Xenograft Rejection by Suppressing Adaptive Immune Cell Response and NF-κB Signaling Activation [ Cell Transplant, 2021, 30:9636897211054503] | PubMed: 34814739 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
