|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C16H17N7O2S |
|||
| Molecular Weight | 371.42 | CAS No. | 1187594-09-7 | |
| Solubility (25°C)* | In vitro | DMSO | 74 mg/mL (199.23 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Baricitinib is a selective JAK1 and JAK2 inhibitor with IC50 of 5.9 nM and 5.7 nM in cell-free assays, ~70 and ~10-fold selective versus JAK3 and Tyk2, no inhibition to c-Met and Chk2. Baricitinib is found to reduce or interrupt the passage of the virus into target cells and is used in the treatment research for COVID-19. Phase 3. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | Baricitinib inhibits IL-6–stimulated phosphorylation of the canonical substrate STAT3 (pSTAT3) and subsequent production of the chemokine MCP-1 with IC50 values of 44 nM and 40 nM, respectively, in PBMCs. Baricitinib also inhibits pSTAT3 stimulated by IL-23 with IC50 od 20 nM in isolated naive T-cells. [1] |
||||||||
| In vivo | Baricitinib inhibits IL-6–stimulated phosphorylation of STAT3 in whole blood with an IC50 of 128 nM. Baricitinib (10 mg/kg p.o.) is expected to inhibit JAK1/2 signaling (by ≥50%) in rats for about 8 hours. Baricitinib (10 mg/mL, p.o.) inhibits disease scores in dose-dependent manner in rats with established disease in the adjuvant arthritis model. Baricitinib treatment, compared with vehicle, inhibits the increase in hind paw volumes during the 2 weeks of treatment by 50% at a dose of 1 mg/kg and >95% at doses of 3 mg/kg or 10 mg/kg. Baricitinib treatment, compared with vehicle, also inhibits composite score of immune infiltrate, edema, and periarticular tissue appearance by 27% at a dose of 1 mg/kg, 64% at doses of 3 mg/kg and 82% at doses of 10 mg/kg in rats with established disease in the adjuvant arthritis model. Baricitinib reduces bone resorption by 15%, 61%, and 67% with increasing dose level (1, 3, and 10 mg/kg) in rats with established disease in the adjuvant arthritis model. Baricitinib (10 mg/kg, daily for 2 wk, p.o.) results in radiographic improvements with restoration of the normal architecture and appearance to the ankle and tarsals in rats with established disease in the adjuvant arthritis model. Baricitinib reduces levels of pSTAT3 in a dose- and time-dependent manner in the peripheral blood of rAIA animals. Baricitinib (10 mg/mL, p.o.) improves a composite score of joint damage by 47% in the murine CIA model. Baricitinib (10 mg/kg) reduces pannus (74%) and bone damage (78%) and improves cartilage damage (43%) and signs of inflammation (33%), resulting in a 53% improvement in an aggregate score of disease in the collagen Ab-induced arthritis (CAIA) murine model. Baricitinib (10 mg/kg) inhibits the delayed-type hypersensitivity response by 48% in both the CIA and CAIA models. [1] Baricitinib is efficacious in active rheumatoid arthritis patients refractory to disease modifying drugs and biologics. [2] Baricitinib preferentially inhibits JAK1 and JAK2, with 10-fold selectivity over Tyk2 and 100-fold over JAK3. The observed effects of GLPG-0634 on the ACR20, albeit in a smaller study, appear to be at least as good as that seen with tofacitinib and superior to that of baricitinib, since baricitinib only moderately affect the ACR20 values in Phase IIa clinical studies. [3] Baricitinib has the dose-limiting side-effect of inducing anaemia which has been attributed to its effects on JAK2 but has clearly shown efficacy. [4] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
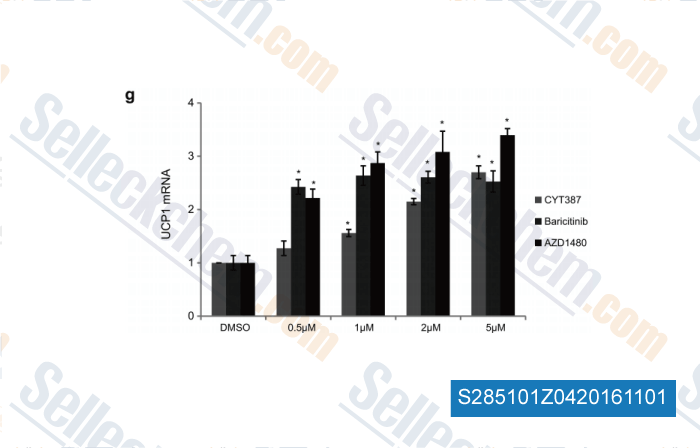
-
, , Nat Cell Biol,2014, 17(1):57-67
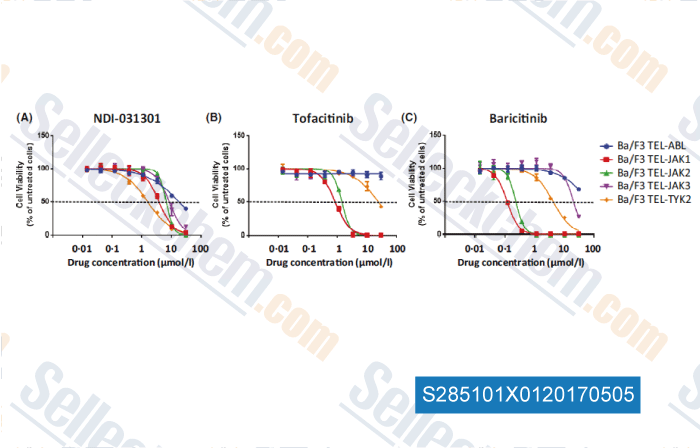
-
Data from [Data independently produced by , , Br J Haematol, 2017, 177(2):271-282]

-
Data from [Data independently produced by , , J Physiol, 2015, 593(24):5269-82]
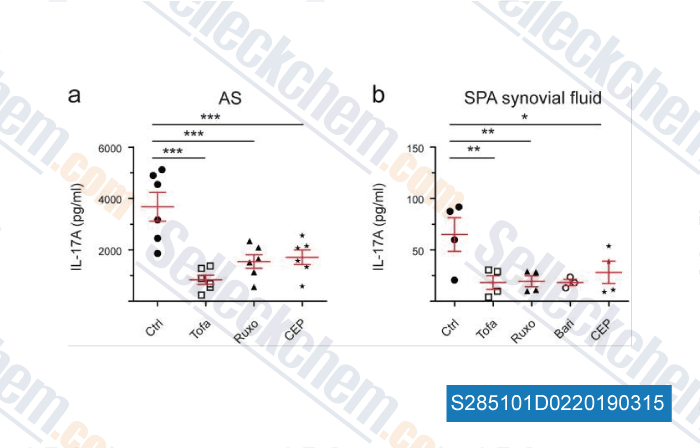
-
Data from [Data independently produced by , , Sci Rep, 2018, 8(1):15645]
Selleck's Baricitinib has been cited by 65 publications
| T-bet+ CXCR3+ B cells drive hyperreactive B-T cell interactions in multiple sclerosis [ Cell Rep Med, 2025, 6(3):102027] | PubMed: 40107244 |
| Spatial proteomics identifies JAKi as treatment for a lethal skin disease [ Nature, 2024, ] | PubMed: 39415009 |
| Macrophage re-programming by JAK inhibitors relies on MAFB [ Cell Mol Life Sci, 2024, 81(1):152] | PubMed: 38528207 |
| Baricitinib inhibits the activation of innate immune cells and exerts therapeutic effects on acute peritonitis and systemic inflammatory response syndrome [ Int Immunopharmacol, 2024, 143(Pt 3):113568] | PubMed: 39488916 |
| A panel of janus kinase inhibitors identified with anti-inflammatory effects protect mice from lethal influenza virus infection [ Antimicrob Agents Chemother, 2024, 68(4):e0135023.] | PubMed: 38470034 |
| Pacritinib inhibits proliferation of primary effusion lymphoma cells and production of viral interleukin-6 induced cytokines [ Sci Rep, 2024, 14(1):4125] | PubMed: 38374336 |
| Novel JAK Inhibitors to Reduce Graft-Versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in a Preclinical Mouse Model [ Molecules, 2024, 29(8)1801] | PubMed: 38675621 |
| Activation of endogenous retroviruses and induction of viral mimicry by MEK1/2 inhibition in pancreatic cancer [ Sci Adv, 2024, 10(13):eadk5386] | PubMed: 38536927 |
| Microglia replacement by ER-Hoxb8 conditionally immortalized macrophages provides insight into Aicardi-Goutières Syndrome neuropathology [ bioRxiv, 2024, 2024.09.18.613629.] | PubMed: 39345609 |
| Interferon signaling promotes tolerance to chromosomal instability during metastatic evolution in renal cancer [ Nat Cancer, 2023, 4(7):984-1000] | PubMed: 37365326 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
