|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C24H28O2 |
|||
| Molecular Weight | 348 | CAS No. | 153559-49-0 | |
| Solubility (25°C)* | In vitro | DMSO | 18 mg/mL (51.72 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Bexarotene is a retinoid specifically selective for retinoid X receptors, used as an oral antineoplastic agent in the treatment of cutaneous T-cell lymphoma. | |
|---|---|---|
| Targets |
|
|
| In vitro | Bexarotene treatment at 1 mM and 10 mM for 96 h increases the number of cells with sub-G1 populations and annexin V binding in a dose-dependent manner compared with vehicle controls (DMSO) in well-established CTCL cell lines (MJ, Hut78, and HH), respectively. Bexarotene treatment suppresses the expression of retinoid X receptor alpha and retinoic acid receptor alpha proteins in all three lines compared with untreated controls. Bexarotene treatment decreases the protein levels of survivin, activates caspase-3, and cleaved poly(ADP-Ribose) polymerase, but has no obvious effect on expression of Fas/Fas ligand and bcl-2 proteins in all three CTCL lines. [1] Bexarotene induces a loss of viability and more pronounced inhibition of clonogenic proliferation in HH and Hut-78 cells, whereas the MJ line exhibits resistance. Bexarotene upregulates and activates Bax in sensitive lines, although not enough to signal significant apoptosis. Bexarotene signals both G(1) and G(2)/M arrest by the modulation of critical checkpoint proteins. Bexarotene activates p53 by phosphorylation at Ser15, which influences the binding of p53 to promoters for cell cycle arrest, induces p73 upregulation, and, in concordance, also modulates some p53/p73 downstream target genes, such as p21, Bax, survivin and cdc2. [2] | |
| In vivo | Bexarotene significantly prevents ER-negative mammary tumorigenesis with less toxicity than naturally occurring retinoids in animal models. Bexarotene inhibits the development of preinvasive mammary lesions such as hyperplasias and carcinoma-in-situ in MMTV-erbB2 mice. [3] |
Protocol (from reference)
References
|
Customer Product Validation
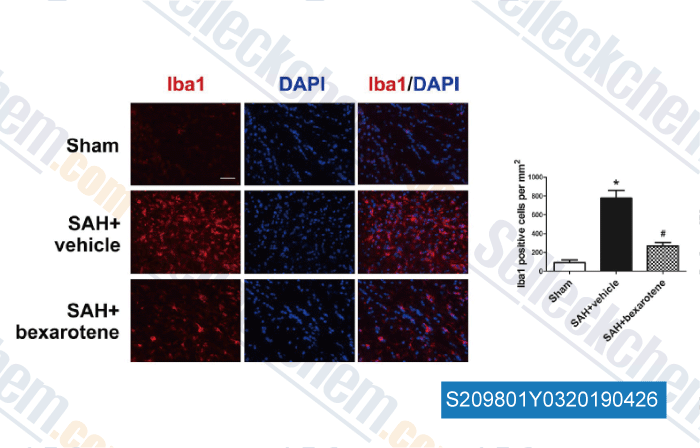
-
Data from [Data independently produced by , , Neurol Res, 2018, 40(8):702-708]
Selleck's Bexarotene has been cited by 15 publications
| Whitening of brown adipose tissue inhibits osteogenic differentiation via secretion of S100A8/A9 [ iScience, 2024, 27(2):108857] | PubMed: 38303710 |
| GZ17-6.02 interacts with bexarotene to kill mycosis fungoides cells [ Oncotarget, 2024, 15:124-133] | PubMed: 38329728 |
| Chromatin accessibility dynamics dictate renal tubular epithelial cell response to injury [ Nat Commun, 2022, 13(1):7322] | PubMed: 36443310 |
| Targeting the TR4 nuclear receptor with antagonist bexarotene can suppress the proopiomelanocortin signalling in AtT-20 cells [ J Cell Mol Med, 2021, 10.1111/jcmm.16074] | PubMed: 33491272 |
| Targeting the TR4 nuclear receptor with antagonist bexarotene can suppress the proopiomelanocortin signalling in AtT-20 cells [ J Cell Mol Med, 2021, 25(5):2404-2417] | PubMed: 33491272 |
| Effect of propylene glycol on the skin penetration of drugs [ Arch Dermatol Res, 2020, 312(5):337-352] | PubMed: 31786711 |
| The support of genetic evidence for cardiovascular risk induced by antineoplastic drugs [ Sci Adv, 2020, 6(42)eabb8543] | PubMed: 33055159 |
| [ Nat Metab, 2019, ] | PubMed: 31984309 |
| Activation of retinoid X receptor by bexarotene attenuates neuroinflammation via PPARγ/SIRT6/FoxO3a pathway after subarachnoid hemorrhage in rats. [ J Neuroinflammation, 2019, 16(1):47] | PubMed: 30791908 |
| Prediction of the skin permeability of topical drugs using in silico and in vitro models. [ Eur J Pharm Sci, 2019, 136:104945] | PubMed: 31163216 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
