|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C45H57NO14 |
|||
| Molecular Weight | 835.93 | CAS No. | 183133-96-2 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (119.62 mM) | |
| Ethanol | 100 mg/mL (119.62 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Cabazitaxel is a semi-synthetic derivative of a natural taxoid that kills cancer cells by inhibiting cell division and growth. Cabazitaxel exerts its effects by inhibiting microtubule growth and assembly, processes that are essential for cells to divide. Cabazitaxel induces autophagy via the PI3K/Akt/mTOR pathway. | |
|---|---|---|
| Targets |
|
|
| In vitro | Cabazitaxel increases CYP3A enzyme activities in rat hepatocytes. The mean ex-vivo human plasma protein binding of Cabazitaxel is 91.6%. Cabazitaxel is rapidly and extensively metabolised in numerous metabolites. Cabazitaxel demonstrates activity in several murine and human resistant cell lines. [1] With a 4-day exposure to cabazitaxel, cytotoxicity is noted with relatively low cabazitaxel concentrations. Cabazitaxel shows high antitumor activity in 3 human colorectal cell lines (HCT-116, HCT-8, and HT-29). [2] |
|
| In vivo | In accompanying models, Cabazitaxel is noted to have significant antitumor activity. In murine tumor xenografts (colon C38 and pancreas P03), Cabazitaxel elicites complete tumor regressions. Using SF-295 and U251 human glioblastoma cell lines, both orthotopic and subcutaneous murine xenografts are generated. Cabazitaxel treatment leads to complete regression in the majority of subcutaneously implanted tumors. Furthermore, in orthotopic models, Cabazitaxel leads to complete tumor regression in 4 out of 10 U251 tumors. [2] |
|
| Features | A semi-synthetic derivative of a natural taxoid. |
Protocol (from reference)
| Cell Assay: |
|
|---|
References
Customer Product Validation
-
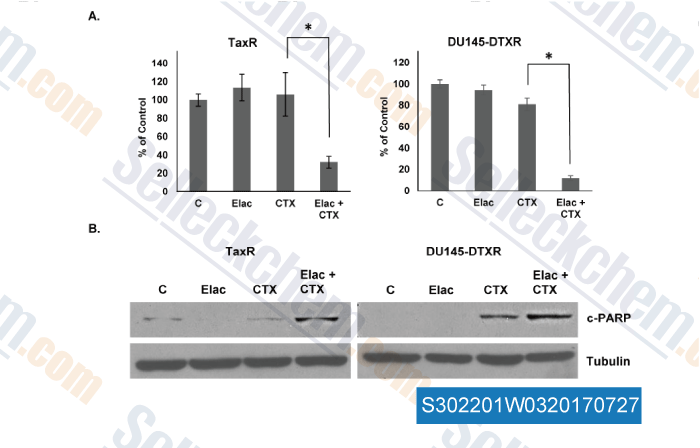
, , Mol Cancer Ther, 2017, 16(10):2257-2266
-
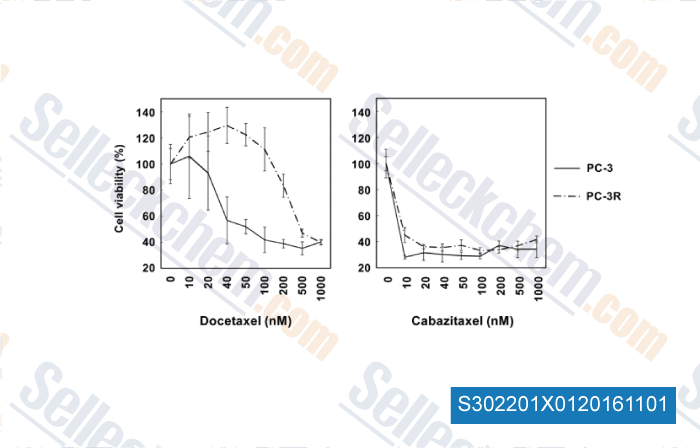
, , Urol Oncol, 2015, 33(9):385.e15-20.
-
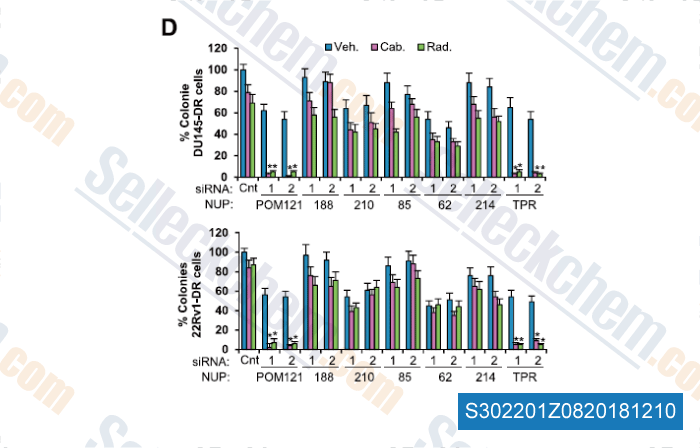
Data from [Data independently produced by , , Cell, 2018, 174(5):1200-1215]
-
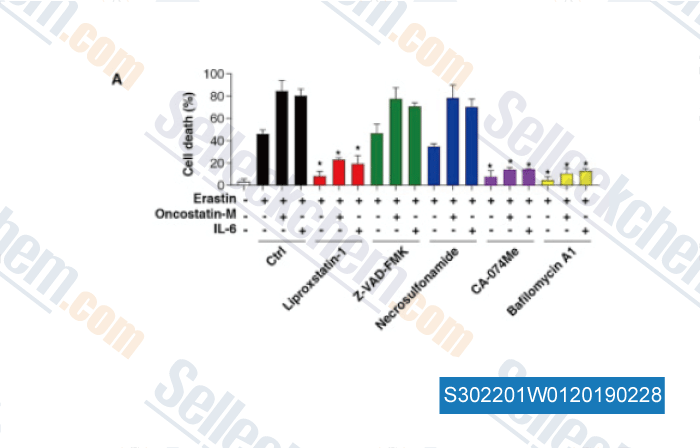
Data from [Data independently produced by , , Clin Cancer Res, 2018, doi:10.1158/1078-0432.CCR-18-0704]
Selleck's Cabazitaxel has been cited by 28 publications
| Fabrication of hyaluronic acid-altered gold complex delivery for head and neck squamous cell carcinoma therapy with high antitumor efficacy and low in vivo toxicity [ J Photochem Photobiol B, 2024, 253:112877] | PubMed: 38484648 |
| Targeting prostate tumor low-molecular weight tyrosine phosphatase for oxidation-sensitizing therapy [ Sci Adv, 2024, 10(5):eadg7887] | PubMed: 38295166 |
| Ritonavir reverses resistance to docetaxel and cabazitaxel in prostate cancer cells with acquired resistance to docetaxel [ Cancer Drug Resist, 2024, 7:3] | PubMed: 38318527 |
| Relevance of the organic anion transporting polypeptide 1B3 (OATP1B3) in the personalized pharmacological treatment of hepatocellular carcinoma [ Biochem Pharmacol, 2023, 214:115681] | PubMed: 37429423 |
| Berbamine targets cancer stem cells and reverses cabazitaxel resistance via inhibiting IGF2BP1 and p-STAT3 in prostate cancer [ Prostate, 2023, 10.1002/pros.24632] | PubMed: 37828768 |
| The therapeutic effect of KSP inhibitors in preclinical models of cholangiocarcinoma [ Cell Death Dis, 2022, 13-9:799] | PubMed: 36123339 |
| Activation of the ABCB1-amplicon promotes cellular viability and resistance to docetaxel and cabazitaxel in castration-resistant prostate cancer [ Mol Cancer Ther, 2021, molcanther.0983.2020] | PubMed: 34326198 |
| Proteomic analysis of extracellular vesicles identified PI3K pathway as a potential therapeutic target for cabazitaxel-resistant prostate cancer [ Prostate, 2021, 10.1002/pros.24138] | PubMed: 33905554 |
| DNA-PKc inhibition overcomes taxane resistance by promoting taxane-induced DNA damage in prostate cancer cells [ Prostate, 2021, 10.1002/pros.24200] | PubMed: 34297853 |
| The Role of Crosstalk between AR3 and E2F1 in Drug Resistance in Prostate Cancer Cells. [ Cells, 2020, 28;9(5)] | PubMed: 32354165 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
