|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C23H28F2N6O4S |
|||
| Molecular Weight | 522.57 | CAS No. | 274693-27-5 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (191.36 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Ticagrelor (AZD6140, AR-C 126532XX) is the first reversibly binding oral P2Y12 receptor antagonist with Ki of 2 nM. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Ticagrelor is an active drug which, does not require metabolic activation after intestinal absorption. It does not compete directly with ADP at the ADP binding site but occupies an adjacent binding site and acts in an allosteric way, resulting in a reversible conformational change of the receptor. Ticagrelor binds reversibly to the receptor and exhibits rapid onset and offset of effect. Binding studies in rh-P2Y12 receptor-transfected CHO-K1 cells indicate that ticagrelor exhibits potent, rapid, and reversible binding, with a Kd of 10.5 nM, a kon (association constant) of 0.00011/(nM•s), a koff (dissociation constant) of 0.00087/s, and half-life values of 4 min for binding and 14 min for unbinding, indicating that the magnitude of platelet inhibition is dependent on concentrations of drug available to bind platelets. [1] Ticagrelor moderately inhibits CYP2C9 activity in human liver microsomes, while exhibiting little or no inhibition of CYP1A2, CYP2B6, CYP2C8, CYP2C19, CYP2D6, and CYP2E1. In human liver microsomes, ticagrelor inhibits midazolam 4-hydroxylation, while activating 1_-hydroxylation of midazolam. Evaluated in fresh human hepatocytes, ticagrelor is not an inducer of CYP1A2 or CYP3A4. [3] |
||
| In vivo | Absorption of ticagrelor is rapid with t max of 1.3-2 h. And the Cmax and area under the plasma concentration-time curve from time 0 to infinity increases in an apparently dose-proportional manner over the dose range studied, indicating linear pharmacokinetics. The mean terminal-phase half-life (t1/2) is approximately 7-8.5 h for ticagrelor. Inhibition of platelet aggregation (IPA) is dose related and is nearly complete at 2 h at doses of 100-400 mg. Ticagrelor is well tolerated, with no serious or doserelated adverse events or notable changes in laboratory values observed. [2] |
||
| Features | First-in-class of a new type of P2Y12 antagonist known as cyclopentyl-triazolo-pyrimidines. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
References
Customer Product Validation
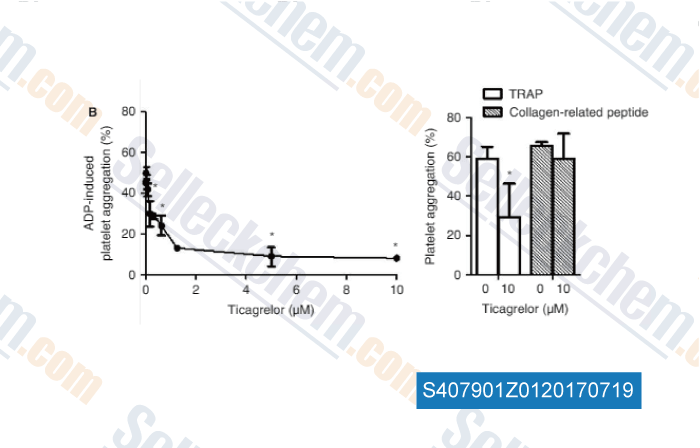
-
Data from [Data independently produced by , , J Thromb Haemost, 2017, 15(6):1191-1202]
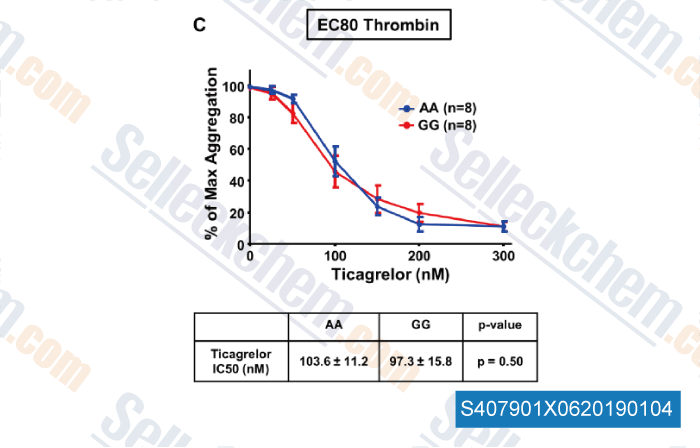
-
Data from [Data independently produced by , , J Thromb Haemost, 2018, doi:10.1111/jth.14318]
Selleck's Ticagrelor has been cited by 9 publications
| Antiplatelet Activity of Tetramethylpyrazine via Regulation of the P2Y12 Receptor Downstream Signaling Pathway [ Evid Based Complement Alternat Med, 2022, 2022:7941039] | PubMed: 35378909 |
| Targeting the P2Y13 Receptor Suppresses IL-33 and HMGB1 Release and Ameliorates Experimental Asthma [ Am J Respir Crit Care Med, 2021, 10.1164/rccm.202009-3686OC] | PubMed: 34860143 |
| Ticagrelor Inhibits the NLRP3 Inflammasome to Protect Against Inflammatory Disease Independent of the P2Y 12 Signaling Pathway [ Cell Mol Immunol, 2020, 10] | PubMed: 32523112 |
| Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by Targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells. [ Cancers (Basel), 2020, 12(1)] | PubMed: 31968611 |
| The Protease‐Activated Receptor 4 Ala120Thr Variant Alters Platelet Responsiveness to Low‐Dose Thrombin, Protease‐Activated Receptor 4 Desensitization and Is Blocked by Noncompetitive P2Y12 Inhibition [Whitley MJ, et al. J Thromb Haemost, 2018, 10.1111/jth.14318] | PubMed: 30347494 |
| Measurement of platelet aggregation, independently of patient platelet count: a flow-cytometric approach. [Vinholt PJ, et al. J Thromb Haemost, 2017, 15(6):1191-1202] | PubMed: 28296243 |
| A Platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. [Chen Y, et al. J Pharm Biomed Anal, 2016, 117:178-83] | PubMed: 26355772 |
| A Platelet/CMC coupled with offline UPLC-QTOF-MS/MS for screening antiplatelet activity components from aqueous extract of Danshen. [Ying Chen, et al. J Pharmaceut Biomed, 2015, 10.1016/j.jpba.2015.06.009] | |
| The importance of sample collection when using single cytokine levels and systemic cytokine profiles as biomarkers-a comparative study of serum versus plasma samples. [Tvedt TH, et al. J Immunol Methods, 2015, 10.1016/j.jim.2015.01.006] | PubMed: 25637409 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
