- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Magnetic Separator
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Betahistine 2HCl
Synonyms: PT-9
Betahistine (PT-9) is a histamine H3 receptor inhibitor with IC50 of 1.9 μM.
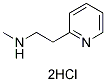
Betahistine 2HCl Chemical Structure
CAS No. 5579-84-0
Purity & Quality Control
Batch:
Purity:
99.93%
99.93
Betahistine 2HCl Related Products
| Related Targets | H1 receptor H2 receptor H3 receptor H4 receptor | Click to Expand |
|---|---|---|
| Related Products | GSK2879552 2HCl JNJ-7777120 Mianserin HCl Ebastine Ciproxifan Maleate | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Neuronal Signaling Compound Library CNS-Penetrant Compound Library Anti-alzheimer Disease Compound Library | Click to Expand |
Signaling Pathway
Biological Activity
| Description | Betahistine (PT-9) is a histamine H3 receptor inhibitor with IC50 of 1.9 μM. | ||
|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | Betahistine progressively enhances cAMP formation with a maximal effect, observed up to 10 nM, in CHO(H3R) cells incubated with 3 μM forskolin. In contrast, at concentrations higher than 10 nM betahistine progressively inhibits cAMP formation in CHO(H3R) cells incubated with 3 μM forskolin. Betahistine progressively reduces A23187-evoked [3H]arachidonic acid release (EC50=0.1 nM) with a maximal effect, observed up to 30 nM A23187-evoked [3H]arachidonic acid release from CHO(H3R) cells. Betahistine progressively enhanced the release of A23187-evoked [3H]arachidonic acid from CHO(H3R) cells at concentrations higher than 30 nM. [1] | |||
|---|---|---|---|---|
| In Vivo | ||
| In vivo | Betahistine (< 30 mg/kg) increases t-MeHA levels in a dose-dependent manner with an ED50 of 2 mg/kg and a maximal effect of ∼35% reached at 30 mg/kg in mouse brain. [1] Betahistine (16 mg twice per day for 3 months) has a significant effect on the frequency, intensity and duration of vertigo attacks, associated symptoms and the quality of life also are significantly improved in patients with Meniere's disease. [2] Betahistine-dihydrochloride (16 mg tid and 48 mg tid) shows that the number of attacks per month decreased in both doses over time in Meni鑢e's disease. [3] Betahistine (50 mg/kg) treatment induces symmetrical changes with up-regulation of histidine decarboxylase mRNA in the tuberomammillary nucleus and reduction of [3H]N-alpha-methylhistamine labeling in both the tuberomammillary nucleus, the vestibular nuclei complex and nuclei of the inferior olive in brain sections of cats. [4] | |
|---|---|---|
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00852956 | Completed | Healthy |
OBEcure Ltd. |
February 2009 | Phase 1 |
| NCT00585585 | Terminated | Recurrent Major Depressive Disorder With Atypical Features |
University of Cincinnati |
July 2007 | Phase 2 |
| NCT00459992 | Completed | Obesity|Overweight|Overnutrition |
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)|National Institutes of Health Clinical Center (CC) |
April 10 2007 | Phase 1 |
Chemical Information & Solubility
| Molecular Weight | 209.12 | Formula | C8H12N2.2HCl |
| CAS No. | 5579-84-0 | SDF | Download Betahistine 2HCl SDF |
| Smiles | CNCCC1=CC=CC=N1.Cl.Cl | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 38 mg/mL ( (181.71 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 38 mg/mL Ethanol : 38 mg/mL |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Betahistine 2HCl | Betahistine 2HCl supplier | purchase Betahistine 2HCl | Betahistine 2HCl cost | Betahistine 2HCl manufacturer | order Betahistine 2HCl | Betahistine 2HCl distributor