- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Magnetic Separator
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Crizotinib hydrochloride
Synonyms: Xalkori, PF-02341066 hydrochloride
Crizotinib (PF-02341066) hydrochloride (Xalkori) inhibits tyrosine phosphorylation of c-Met and nucleophosmin (NPM)-anaplastic lymphoma kinase (ALK) with IC50 of of 11 nM and 24 nM in cell-based assays, respectively. Crizotinib hydrochloride is also a potent ROS1 inhibitor with Ki less than 0.025 nM. Crizotinib induces autophagy through inhibition of the STAT3 pathway in multiple lung cancer cell lines.
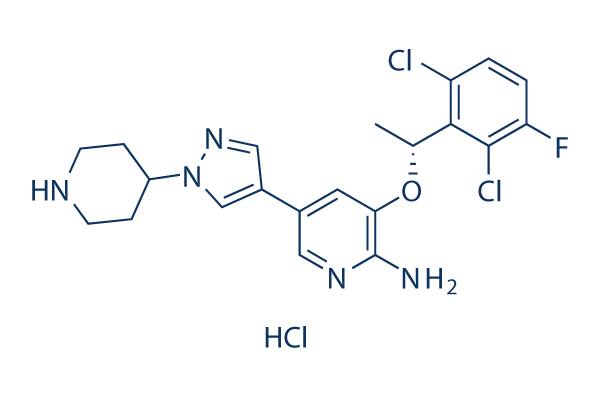
Crizotinib hydrochloride Chemical Structure
CAS No. 1415560-69-8
Purity & Quality Control
Batch:
Purity:
99.82%
99.82
Crizotinib hydrochloride Related Products
| Related Products | SGX-523 Foretinib PHA-665752 SU11274 BMS-777607 JNJ-38877605 Tivantinib PF-04217903 Tepotinib Savolitinib (AZD6094) MGCD-265 analog Golvatinib (E7050) Merestinib (LY2801653) MK-2461 NPS-1034 AMG 337 Sodium L-ascorbyl-2-phosphate NVP-BVU972 BMS-794833 S49076 | Click to Expand |
|---|---|---|
| Related Compound Libraries | Kinase Inhibitor Library Tyrosine Kinase Inhibitor Library PI3K/Akt Inhibitor Library Cell Cycle compound library Angiogenesis Related compound Library | Click to Expand |
Signaling Pathway
Biological Activity
| Description | Crizotinib (PF-02341066) hydrochloride (Xalkori) inhibits tyrosine phosphorylation of c-Met and nucleophosmin (NPM)-anaplastic lymphoma kinase (ALK) with IC50 of of 11 nM and 24 nM in cell-based assays, respectively. Crizotinib hydrochloride is also a potent ROS1 inhibitor with Ki less than 0.025 nM. Crizotinib induces autophagy through inhibition of the STAT3 pathway in multiple lung cancer cell lines. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | PF-2341066 displays similar potency against c-Met phosphorylation in mIMCD3 mouse or MDCK canine epithelial cells with IC50 of 5 nM and 20 nM, respectivly. PF-2341066 shows improved or similar activity against NIH3T3 cells engineered to express c-Met ATP-binding site mutants V1092I or H1094R or the P-loop mutant M1250T with IC50 of 19 nM, 2 nM and 15 nM, respectively, compared with NIH3T3 cells expressing wild-type receptor with IC50 of 13 nM. In contrast, a marked shift in potency of PF-2341066 is observed against cells engineered to express c-Met activation loop mutants Y1230C and Y1235D with IC50 of 127 nM and 92 nM, respectively, compared with wild-type receptor. PF-2341066 also potently prevents the phosphorylation of c-Met in NCI-H69 and HOP92 cells, with IC50 of 13 nM and 16 nM, respectively, which express the endogenous c-Met variants R988C and T1010I, respectively. PF-2341066 is >1,000-fold selective for the VEGFR2 and PDGFRβ RTKs, >250-fold selective for IRK and Lck, and ∼40- to 60-fold selective for Tie2, TrkA, and TrkB, all compared with c-Met. PF-2341066 is 20- to 30-fold selective for RON and Axl RTKs. In contrast, PF-2341066 shows a near-equivalent IC50 of 24 nM against the nucleophosmin (NPM)-anaplastic lymphoma kinase (ALK) oncogenic fusion variant of the ALK RTK expressed by the KARPAS299 human anaplastic large cell lymphoma (ALCL) cell line. PF-2341066 inhibits c-Met–dependent neoplastic phenotypes of cancer cells and angiogenic phenotypes of endothelial cells. PF-2341066 suppresses human GTL-16 gastric carcinoma cell growth with IC50 of 9.7 nM. PF-2341066 induces apoptosis in GTL-16 cells with IC50 of 8.4 nM. PF-2341066 inhibits HGF-stimulated human NCI-H441 lung carcinoma cell migration and invasion with IC50 of 11 nM and 6.1 nM, respectively. PF-2341066 inhibits MDCK cell scattering with IC50 of 16 nM. PF-2341066 prevents HGF-stimulated c-Met phosphorylation, cell survival, and Matrigel invasion with IC50 of 11 nM, 14 nM and 35 nM, respectively. In addition, PF-2341066 prevents serum-stimulated HMVEC branching tubulogenesis (formation of vascular tubes) in fibrin gels.[4] |
|||
|---|---|---|---|---|
| Kinase Assay | Cellular kinase phosphorylation ELISA assays | |||
| Cells are seeded in 96-well plates in media supplemented with 10% fetal bovine serum (FBS) and transferred to serum-free media [with 0.04% bovine serum albumin (BSA)] after 24 h. In experiments investigating ligand-dependent RTK phosphorylation, corresponding growth factors are added for up to 20 min. After incubation of cells with PF-2341066 for 1 h and/or appropriate ligands for the designated times, cells are washed once with HBSS supplemented with 1 mM Na3VO4, and protein lysates are generated from cells. Subsequently, phosphorylation of selected protein kinases is assessed by a sandwich ELISA method using specific capture antibodies used to coat 96-well plates and a detection antibody specific for phosphorylated tyrosine residues. Antibody-coated plates are (a) incubated in the presence of protein lysates at 4°C overnight; (b) washed seven times in 1% Tween 20 in PBS; (c) incubated in a horseradish peroxidase–conjugated anti–total-phosphotyrosine (PY-20) antibody (1:500) for 30 min; (d) washed seven times again; (e) incubated in 3,3′,5,5′-tetramethyl benzidine peroxidase substrate to initiate a colorimetric reaction that is stopped by adding 0.09 N H2SO4; and (f) measured for absorbance in 450 nm using a spectrophotometer. | ||||
| Cell Research | Cell lines | GTL-16 gastric carcinoma cells and T47D breast carcinoma cells | ||
| Concentrations | 0-256 nM | |||
| Incubation Time | 1 h | |||
| Method | Cells including GTL-16 gastric carcinoma cells and T47D breast carcinoma cells are seeded in 96-well plates in media supplemented with 10% fetal bovine serum (FBS) and transferred to serum-free media [with 0.04% bovine serum albumin (BSA)] after 24 hours. In experiments investigating ligand-dependent RTK phosphorylation, corresponding growth factors are added for up to 20 minutes. After incubation of cells with PF-2341066 for 1 hour and/or appropriate ligands for the designated times, cells are washed once with HBSS supplemented with 1 mM Na3VO4, and protein lysates are generated from cells. Subsequently, phosphorylation of selected protein kinases is assessed by a sandwich ELISA method using specific capture antibodies used to coat 96-well plates and a detection antibody specific for phosphorylated tyrosine residues. Antibody-coated plates are (a) incubated in the presence of protein lysates at 4 °C overnight; (b) washed seven times in 1% Tween 20 in PBS; (c) incubated in a horseradish peroxidase–conjugated anti–total-phosphotyrosine (PY-20) antibody (1:500) for 30 min; (d) washed seven times again; (e) incubated in 3,3′,5,5′-tetramethyl benzidine peroxidase substrate to initiate a colorimetric reaction that is stopped by adding 0.09 N H2SO4; and (f) measured for absorbance in 450 nm using a spectrophotometer. |
|||
| In Vivo | ||
| In vivo | In the GTL-16 model, PF-2341066 reveals the ability to cause marked regression of large established tumors (>600 mm3) in both the 50 mg/kg/day and 75 mg/kg/day treatment cohorts, with a 60% decrease in mean tumor volume over the 43-day administration schedule. In an another study, PF-2341066 displays the ability to completely inhibits GTL-16 tumor growth for >3 months, with only 1 of 12 mice exhibiting a significant increase in tumor growth over the 3-month treatment schedule at 50 mg/kg/day. In the NCI-H441 NSCLC model, a 43% decrease in mean tumor volume is observed at 50 mg/kg/day during the 38-day PF-2341066 administration cycle. In the Caki-1 RCC model, a 53% decrease in mean tumor volume is observed to be associated with decreased volume of each tumor by at least 30% at 50 mg/kg/day during the 33-day PF-2341066 administration cycle. PF-2341066 also reveals near-complete prevention of the growth of established tumors at 50 mg/kg/day in the U87MG glioblastoma or PC-3 prostate carcinoma xenograft models, with 97% or 84% inhibition on the final study day, respectively. In contrast, PF-2341066 p.o. given at 50 mg/kg/day does not significantly inhibit tumor growth in the MDA-MB-231 breast carcinoma model, or the DLD-1 colon carcinoma model. A significant dose-dependent reduction of CD31–positive endothelial cells is observed at 12.5 mg/kg/day, 25 mg/kg/day, and 50 mg/kg/day in GTL-16 tumors, indicating that inhibition of MVD shows a dose-dependent correlation to antitumor efficacy. PF-2341066 displays a significant dose-dependent reduction of human VEGFA and IL-8 plasma levels in both the GTL-16 and U87MG models. Marked inhibition of phosphorylated c-Met, Akt, Erk, PLCλ1, and STAT5 levels is observed in GTL-16 tumors following p.o. administration of PF-2341066.[4]
|
|
|---|---|---|
| Animal Research | Animal Models | Female or male nu/nu mice bearing NCI-H441,or DLD-1, or MDA-MB-231 |
| Dosages | 12.5 mg/kg/day, 25 mg/kg/day, and 50 mg/kg/day | |
| Administration | p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06062810 | Not yet recruiting | Non-Small Cell Lung Cancer |
Han Xu M.D. Ph.D. FAPCR Sponsor-Investigator IRB Chair|Medicine Invention Design Inc |
March 28 2024 | Phase 2|Phase 3 |
| NCT04148066 | Completed | Carcinoma Non-Small-Cell Lung |
The Netherlands Cancer Institute|Roche Pharma AG |
July 17 2019 | Not Applicable |
| NCT03947385 | Recruiting | Metastatic Uveal Melanoma|Cutaneous Melanoma|Colorectal Cancer|Other Solid Tumors |
IDEAYA Biosciences |
June 28 2019 | Phase 1|Phase 2 |
| NCT03672643 | Terminated | ALK or ROS1-positive NSCLC |
Pfizer |
January 28 2019 | Phase 4 |
| NCT03439215 | Unknown status | Carcinoma Non-Small-Cell Lung |
Fondazione Ricerca Traslazionale|Clinical research technology Srl |
June 13 2017 | Phase 2 |
Chemical Information & Solubility
| Molecular Weight | 486.8 | Formula | C21H23Cl3FN5O |
| CAS No. | 1415560-69-8 | SDF | -- |
| Smiles | Cl.CC(OC1=C(N)N=CC(=C1)C2=C[N](N=C2)C3CCNCC3)C4=C(Cl)C=CC(=C4Cl)F | ||
| Storage (From the date of receipt) | 3 years -20°C powder | ||
|
In vitro |
DMSO : 97 mg/mL ( (199.26 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 97 mg/mL Ethanol : 97 mg/mL |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Crizotinib hydrochloride | Crizotinib hydrochloride supplier | purchase Crizotinib hydrochloride | Crizotinib hydrochloride cost | Crizotinib hydrochloride manufacturer | order Crizotinib hydrochloride | Crizotinib hydrochloride distributor