
- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-DYKDDDDK Tag magnetic beads
- Anti-DYKDDDDK Tag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Brefeldin A (BFA)
Synonyms: Cyanein, Decumbin
Brefeldin A (BFA) is a lactone antibiotic and ATPase inhibitor for protein transport with IC50 of 0.2 μM in HCT 116 cells, induces cancer cell differentiation and apoptosis. It could also improve the HDR(homology-directed repair) efficiency and be an enhancer of CRISPR-mediated HDR. Brefeldin A is also an inhibitor of autophagy and mitophagy.
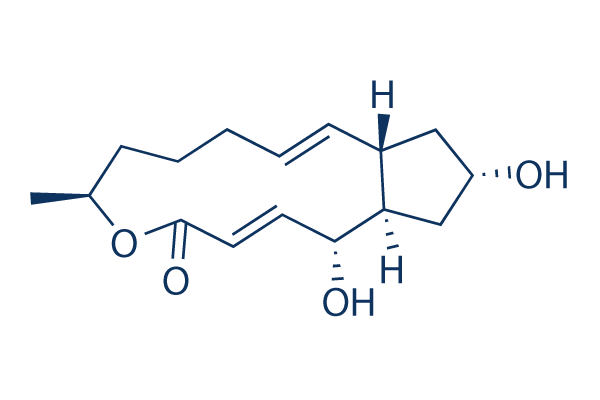
Brefeldin A (BFA) Chemical Structure
CAS: 20350-15-6
Selleck's Brefeldin A (BFA) has been cited by 68 publications
Purity & Quality Control
Batch:
Purity:
99.94%
99.94
Other ATPase Products
Related compound libraries
Choose Selective ATPase Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| PC12 | Function Assay | 2 μM | 1 h | inhibits the L-DOPA (20 μM)-induced transient ERK1/2 phosphorylation | 26363191 | |
| C2C12 | Function Assay | 1 μg/ml | 1 h | abolishes cytokine release from C2C12 myotubes | 26291279 | |
| MEFs WT | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| MEFs VAMP7 KO | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | shows a trend towards a higher concentration of the ER/SR network in the perinuclear area | 26172080 |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | causes a transient Ca2+ release from the ER/SR | 26172080 |
| HEMC-1 | Function Assay | 0.1 µg/ml | 24 h | causes a higher inhibitory effect on exocytosis than nocodazole | 25972759 | |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | abolishes hypoxia-induced release of ATP from apical and basolateral surfaces | 25956988 |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | increases the number and intensity of fluorescent areas especially in perinuclear space | 25956988 |
| Caco-2 | Function Assay | 2.5 μM | 30 min | attenuates the TGF-β1-mediated increase in SERT function | 25954931 | |
| NRK | Function Assay | 200 ng/ml | 4 h | DMSO | rescues mitotic progression | 25948586 |
| HeLa | Function Assay | 200 ng/ml | 3 h | DMSO | induces the artificial break-up of the Golgi complex | 25948586 |
| COS | Function Assay | 1 μg/ml | 3 h | completely disperses the AP-1 signal | 25915900 | |
| DF1 | Function Assay | 1 μM | 48 h | DMSO | disperses the exogenous CSGalNAcT2 protein | 25807054 |
| nHDFs | Function Assay | 1 μM | 2 h | prevents the assembly of cytosolic coat proteins onto Golgi membranes | 25772616 | |
| FRT | Function Assay | 5 μg/ml | 2 h | blocks trafficking through the Golgi complex by inhibiting ER-to-Golgi transport | 25767115 | |
| FRT | Function Assay | 5 μg/ml | 2 h | prevents the increase in cleaved α subunits when [Na+]i was reduced | 25767115 | |
| HepG2 | Function Assay | 1 µM | 24 h | DMSO | decreases the level of PXR mRNA | 25616597 |
| SMCs | Function Assay | 1μg/mL | 3 h | accumulates CNPY2 protein in the ER compartment and no longer co-localized with the Golgi marker | 25589425 | |
| OB-6 | Apoptosis Assay | 2.7 μM | 48 h | induces apoptosis | 25532480 | |
| iPSC-CMs | Function Assay | 500 ng/ml | 48 h | increases the intensity of the higher mobility LAMPs at the cost of the lower mobility species | 25488666 | |
| SP-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| PEXEL-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| H1299 | Function Assay | 10 μg/ml | 24 h | induces autophagy | 25388970 | |
| MDA-MB-231 | Cell Viability Assay | 0–50 μg/mL | 48 h | EC50 = 0.016 µg/mL | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.1 μg/mL | 4 h | induces apoptosis | 25356567 | |
| MDA-MB-231 | Growth Inhibition Assay | 0.01/0.05 μg/mL | 24 h | increases the fraction of sub-G1 cell debris | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.05–1 μg/mL | 24 h | induces PARP (poly ADP-ribose polymerase-1) cleavage | 25356567 | |
| MDA-MB-231 | Function Assay | 0–50 μg/mL | 24 h | inhibits the formation of 3D and 2D colonies | 25356567 | |
| A172 | Function Assay | 10 μg/ml | 4 h | DMSO | results in the retrograde transport of fluorescent granules | 25239507 |
| KMS-6 | Function Assay | 1 μM | 24 h | exhibits half the secretion of galanin-LI as did the control | 25229126 | |
| MEC | Function Assay | 1 μM | 1.5 h | causes a dramatic decrease in the surface VEGFR2 | 25228815 | |
| HEK293/hERG | Function Assay | 10 μM | 1 h | results in a time-dependent reduction mature hERG protein | 25218469 | |
| RBE4 | Apoptosis Assay | 2 μM | 3–24 h | induces apoptosis time dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the XBP1 protein levels after 3 and 6 h of treatment | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases active caspase-12 in a time-dependent manner | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the levels of ROS time-dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces a delayed depletion of the ER Ca2+ content at 6 h of incubation significantly | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces an overload of Ca2+ in the mitochondria in the first 6 h of incubation (p < 0.001) but Ca2+ levels in this organelle decreased after 12 h of incubation | 25128025 | |
| Huh-7 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| HepG2 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| H838-LKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the levels of phosphorylated eIF2α (phospho-eIF2α) | 25011082 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | mimics the effects of insulin and causes robust phosphorylation of Akt (Ser 473) and phosphorylation of AS160 (Thr 642 and Ser 588) | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | recapitulates insulin action with respect to regulating Akt activity and AS160 phosphorylation | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | causes reversible redistribution of GLUT4 | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes redistribution of GLUT4 but not increase in glucose uptake | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes phosphorylation of the FoxO1 transcription factor | 24843827 | |
| HeLa | Function Assay | 5 μg/ml | 3 h | causes nuclear exclusion of the FoxO1 transcription factor and decreases transcription of FoxO1-regulated genes | 24843827 | |
| HEK293 | Function Assay | 5 μg/ml | 12 h | abolishes CMA-induced CRELD2 secretion | 24687431 | |
| COS-1 | Function Assay | 5 µg/ml | 24 h | restricts localization of NB in the perinuclear region | 24671751 | |
| PRP | Function Assay | 10 μM | abrogates SDF-1α-mediated CXCR7 externalization | 24668750 | ||
| RAW264.7 | Apoptosis Assay | 4 μM | 48 h | attenuates the inhibition of ox-LDL-induced apoptosis and the facilitation of cholesterol efflux by Ac-hE-18A-NH2 | 24639032 | |
| MDMs | Apoptosis Assay | 10 μg/ml | 12/15 h | induces apoptosis | 24556695 | |
| PMHs | Function Assay | 10–20 μg/ml | 24 h | DMSO | induced ER stress | 24407242 |
| PMHs | Apoptosis Assay | 10–20 μg/ml | 24 h | DMSO | increases cell death | 24407242 |
| HEK293/tau | Function Assay | 5 μM | 1/2/4 h | induces Golgi fragmentation | 24368089 | |
| HEK293/tau | Function Assay | 5 μM | 3 h | induces tau hyperphosphorylation | 24368089 | |
| ADF | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| U373 | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| RKO-HIPK2i | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| ADF | Function Assay | 10 μM | 6 h | impairs the DC activation | 24228232 | |
| Huh7 | Function Assay | 5 μg/ml | 4 h | abolishes the secretion of intracellular ApoB | 24100140 | |
| Huh7 | Function Assay | 5 μg/ml | 1 h | causes a significant increase in ApoB-crescents | 24100140 | |
| Huh7 | Function Assay | 5–10 ng/ml | 12 h | increases ApoB-crescents without inhibiting secretion | 24100140 | |
| BAECs | Function Assay | 5 μg/ml | 0-4 h | induces the rapid dephosphorylation of eNOS at Ser1179 | 24085225 | |
| Macrophages | Function Assay | 71 µM | 6 h | inhibits lunasin internalization | 24039740 | |
| Colo 205 | Growth Inhibition Assay | 0-5 μg/mL | 48 h | inhibits cell growth in suspension cultures with an estimated IC50 of ~15 ng/mL | 23973996 | |
| Colo 205 | Function Assay | 0.012-0.025 μg/mL | 14 d | reduces the clonogenicity of Colo 205 CSCs | 23973996 | |
| Colo 205 | Apoptosis Assay | 0.1 μg/mL | 0-24 h | induces apoptosis of Colo 205 cells in suspension cultures | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | induces the expression of ER stress-related genes | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | inhibits the activity of MMPs | 23973996 | |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | disrupts the ERGIC and Golgi | 23963534 |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | enhances FMDV infection | 23963534 |
| HeLa | Function Assay | 2 μM | 2 h | attenuates the TNF-induced secretion of IL-15 | 23950892 | |
| HFS | Function Assay | 0-1 μg/ml | 24 h | GLTP expression reaches a plateau at concentrations as low as 0.01 µg/ml | 23894633 | |
| HFS | Function Assay | 0.01 µg/ml | 24 h | increases the expression of glycosphingolipid synthase genes at 6 h | 23894633 | |
| OVCAR-3 | Growth Inhibition Assay | 1–15 μM | 24 h | induces a loss of cell viability dose dependently | 23826964 | |
| OVCAR-3 | Function Assay | 1–15 μM | 24 h | induces nuclear damage | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 1-10 μM | 4 h | induces the activation of apoptosis-related proteins | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 10 μM | 24 h | induces activation of caspases | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces disruption of the mitochondrial transmembrane potential | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces formation of reactive oxygen species | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | inhibits cell adhesion and migration | 23826964 | |
| MKN45 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| LOVO | Growth Inhibition Assay | IC50=0.12 μg/ml | 23793342 | |||
| A549 | Growth Inhibition Assay | IC50=0.04 μg/ml | 23793342 | |||
| MDA-MB-435 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HepG2 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HL-60 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| neural precursor cells | Function assay | Inhibition of neurosphere proliferation of mouse neural precursor cells by MTT assay | 17417631 | |||
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker betaCoP in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker KDEL in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid AP-1 dispersal from golgi membranes at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid GGA3 dispersal from trans golgi network at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV deleted mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV to AAA mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as decrease in Arf1-GTP levels at 10 ug/ml after 1 hr | 19182783 | |
| Vero | Function assay | 10 uM | Inhibition of GBF1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 uM by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid COPI redistribution from golgi at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as tubule formation from trans golgi network and endosomes before its dispersal at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as giantin positive punctate structures in contact with Sec31-positive ER exit site at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | Inhibition of Arf1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 ug/ml by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Induction of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| NRK | Function assay | 7 uM | 60 mins | Golgi-disturbing activity in golgi apparatus of rat NRK cells assessed as fusion of golgi membrane fusion with endoplasmic reticulum at 7 uM after 60 mins by Hoechst 3342 staining-based immunofluorescence microscopy | 20189813 | |
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, GI50=0.0206μM. | 23805957 | |||
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, TGI=3.48μM. | 23805957 | |||
| HeLa R19 | Antiviral assay | 0.5 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 0.5 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| HeLa | Function assay | 5 uM | 30 to 60 mins | Induction of golgi apparatus disassembly in human HeLa cells at 5 uM after 30 to 60 mins by confocal microscopic analysis | 23805957 | |
| Arabidopsis thaliana root cells | Function assay | 90 uM | 30 mins | Induction of morphological changes of golgi apparatus in Arabidopsis thaliana root cells expressing ST-YFP/VHAa1-RFP at 90 uM after 30 mins by confocal laser scanning microscopic analysis | 23805957 | |
| HeLa R19 | Antiviral assay | 5 to 50 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 5 to 50 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| PC3 | Function assay | 50 nM | 72 hrs | Potentiation of 3 nM docetaxel-induced cytotoxicity against human PC3 cells assessed as decrease in cell viability at 50 nM after 72 hrs by trypan blue exclusion assay | 28462831 | |
| L02 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human L02 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50<0.0004μM. | 28494251 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 28494251 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTT assay, IC50=0.16μM. | 28494251 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50=0.35μM. | 28494251 | ||
| LO2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LO2 cells after 72 hrs by MTT assay, IC50<0.001μM. | 29524728 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50=0.024μM. | 29524728 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50=0.025μM. | 29524728 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 29524728 | ||
| Bel7402/5-FU | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402/5-FU cells after 72 hrs by MTT assay, IC50=0.82μM. | 29524728 | ||
| HeLa | Function assay | 18 uM | 3 hrs | Inhibition of alkaline phosphatase secretion in human HeLa cells at 18 uM incubated for 3 hrs | 31421965 | |
| VERO-E6 | Function assay | 48 hrs | Determination of IC50 values for inhibition of SARS-CoV-2 induced cytotoxicity of VERO-E6 cells after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging, IC50=0.02μM. | ChEMBL | ||
| VERO-E6 | Function assay | 48 hrs | Toxicity CC50 against VERO-E6 cells determined at 48 hours by high content imaging (same conditions as 2_LEY without exposure to 0.01 MOI SARS CoV-2 virus), CC50=0.06μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Brefeldin A (BFA) is a lactone antibiotic and ATPase inhibitor for protein transport with IC50 of 0.2 μM in HCT 116 cells, induces cancer cell differentiation and apoptosis. It could also improve the HDR(homology-directed repair) efficiency and be an enhancer of CRISPR-mediated HDR. Brefeldin A is also an inhibitor of autophagy and mitophagy. | ||
|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | Brefeldin A is a fungal metabolite and blocks the forward transport between the endoplasmic reticulum and Golgi apparatus, Brefeldin A causes an impaired distribution of the membrane proteins. When HCT 116 human colon cancer cell is treated with Brefeldin A, morphological changes indicating cell differentiation are observed. Brefeldin A exerts its cytotoxic effects mainly by inducing differentiation and apoptosis in tumor cells. [1] The treatment of the strips with 20 μg/mL Brefeldin A for 6 hours completely abolishes the relaxation induced by bradykinin in the presence of 10mM indomethacin and 30 μM L-NOARG. The treatment with 20 μg/mL Brefeldin A substantially abolishes the bradykinin-induced decreases in [Ca2+]i and tension in the range of concentrations between 1 nM and 1 mM. Brefeldin A has no effect on the [Ca2+]i elevation in endothelial cells induced by bradykinin or substance P. [2] Addition of the fungal metabolite Brefeldin A does not affect the spontaneous phospholipid-dependent GTPS binding to myr-rARF1 but totally abolishs the retinal isotonic extract (RIE)-catalyzed exchange, with half-maximal inhibition at 2 μM Brefeldin A. Brefeldin A prevents a wide variety of membrane traffic pathways. Brefeldin A inhibits an ADP-ribosylation factor-specific guanine nucleotide exchange activity present in Golgi membranes or in brain cytosol. The complete prevention by Brefeldin A strongly suggests that the retinal extract contains an ARF-specific guanine nucleotide exchange factor. Retinal isotonic extract (RIE)-catalyzed GTPS release from both ADP-ribosylation factors (ARFs) is only partly inhibited by Brefeldin A, even at 300 μM. [3] Brefeldin A induces fusion of the Golgi apparatus with the ER. Brefeldin A abolishes the inhibitory effect of the CERT inhibitor HPA-12. Brefeldin A treatment, which induces fusion of the Golgi apparatus and the ER, rescues the limonoid-induced prevention of sphingomyelin biosynthesis. BFA treatment of CHO cells causes a 2 to 3 fold increase in sphingomyelin synthesis. [4] Apart from B-CLL cells, Brefeldin A reportedly causes apoptosis in multiple myeloma (U266, NCI-H929), Jurkat, HeLa, leukaemia (HL60, K562, BJAB), colon (HT-29) and prostate, as well as adenoid cystic sarcoma cells. The administration of 25 ng/mL of Brefeldin A completely blocks growth of HF4.9 and HF28RA cells, whereas higher Brefeldin A doses (75 ng/mL) are required to achieve the same effect in HF1A3 cells. Cell proliferation is inhibited within 24 hours in a dose-dependent manner and, depending on the cell line, almost complete cessation of 3H-thymdine incorporation is observed at 50-75 ng/mL of Brefeldin A (26%, 76%, 87% inhibition at 50 ng/ml and 75%, 87%, 92% inhibition at 75 ng/mL for HF1A3, HF4.9 and HF28RA cells respectively. Brefeldin A-induced cell killing is in a dose-dependent manner using YO-PRO 1/PI assay. [5] Brefeldin A could improve the HDR(homology-directed repair) efficiency. It is an enhancer of CRISPR-mediated HDR[6]. |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | Human follicular lymphoma cell lines HF1A3, HF4.9 and HF28RA | ||
| Concentrations | 0 ng/mL -75 ng/mL | |||
| Incubation Time | 5 days | |||
| Method | HF1A3, HF4.9 cell viability upon the treatments is tested using double staining of cells with YO-PRO 1/PI and SYTO16/PI probes. To access cell proliferation, cells are treated with 0–100 ng/mL Brefeldin A in complete medium for 20 hours before adding 1 μCi/mL [methyl-3H]-thymidine for additional 4 hours at 37 °C. The incorporated radioactive thymidine is quantified by scintillation counting with Microbeta counter. To examine long-term effects of Brefeldin A treatment, cells are seeded at initial concentration 105 cells/mL and treated with 0-75 ng/mL Brefeldin A for up to 5 days. At the time indicated, a sample of cells is removed and viable cell number is assessed by standard Trypan Blue exclusion assay. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p53 / GRP78 |
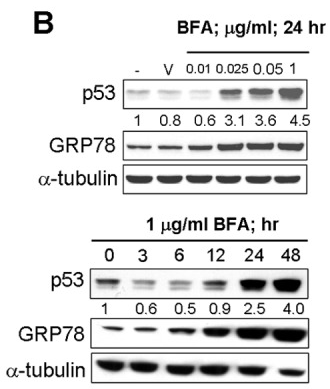
|
22859938 | |
| Immunofluorescence | FMNL1 / GM130 ErbB3 / Calnexin MTP / GBF1 |
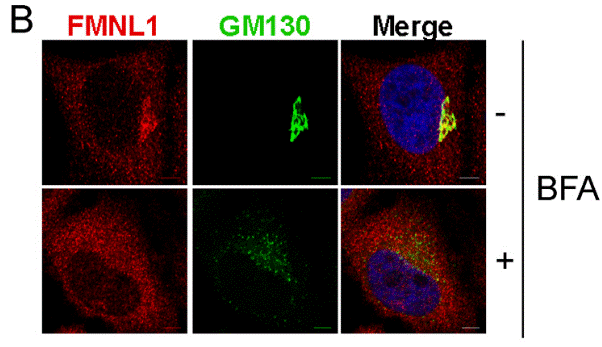
|
21868368 | |
| Growth inhibition assay | Cell viability |
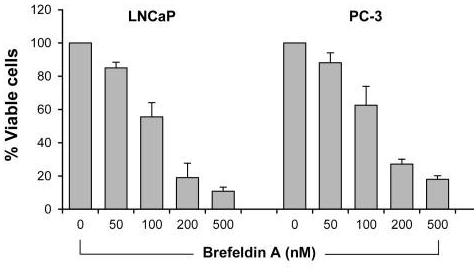
|
28462831 | |
| In Vivo | ||
| In vivo |
Brefeldin A (BFA) is a lactone antibiotic and a specific inhibitor of protein trafficking. Brefeldin A blocks the transport of secreted and membrane proteins from endoplasmic reticulum to Golgi apparatus. |
|
|---|---|---|
| Animal Research | Animal Models | C57BL/6 mice |
| Dosages | 250 µg | |
| Administration | i.p. | |
Chemical lnformation & Solubility
| Molecular Weight | 280.36 | Formula | C16H24O4 |
| CAS No. | 20350-15-6 | SDF | Download Brefeldin A (BFA) SDF |
| Smiles | CC1CCCC=CC2CC(CC2C(C=CC(=O)O1)O)O | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 56 mg/mL ( (199.74 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Brefeldin A (BFA) | Brefeldin A (BFA) supplier | purchase Brefeldin A (BFA) | Brefeldin A (BFA) cost | Brefeldin A (BFA) manufacturer | order Brefeldin A (BFA) | Brefeldin A (BFA) distributor







































