
- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-DYKDDDDK Tag magnetic beads
- Anti-DYKDDDDK Tag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
TMZ(Temozolomide)
Synonyms: NSC 362856,CCRG 81045,Methazolastone
TMZ(Temozolomide) is a monofunctional SN-1 alkylating agent that can modify nitrogen atoms in the DNA ring and the extracyclic oxygen group, chemically converted to MTIC and degrades to methyldiazonium cation, which transfers methyl groups to DNA at physiologic pH. A DNA damage inducer in L-1210 and L-1210/BCNU cells. Temozolomide induces apoptosis and exhibits antitumor activity.
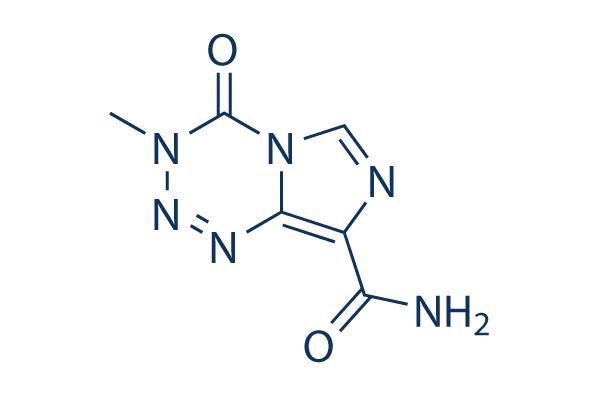
TMZ(Temozolomide) Chemical Structure
CAS: 85622-93-1
Selleck's TMZ(Temozolomide) has been cited by 198 publications
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Other DNA/RNA Synthesis Products
Related compound libraries
Choose Selective DNA/RNA Synthesis Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Kelly | Growth Inhibition Assay | 48 h | IC50=139.20 ± 5.95 μM | 25960282 | ||
| KellyCis83 | Growth Inhibition Assay | 48 h | IC50=251.00 ± 15.75 μM | 25960282 | ||
| SK-N-AS | Growth Inhibition Assay | 48 h | IC50=227.70 ± 22.15 μM | 25960282 | ||
| SK-N-ASCis24 | Growth Inhibition Assay | 48 h | IC50=480.60 ± 101.15 μM | 25960282 | ||
| CHP-212 | Growth Inhibition Assay | 48 h | IC50=7.97 ± 0.69 μM | 25960282 | ||
| CHP-212Cis100 | Growth Inhibition Assay | 48 h | IC50=9.55 ± 0.88 μM | 25960282 | ||
| U87 | Function Assay | 100 μM | 24-72 h | induces DcR1 expression | 25808868 | |
| LN229 | Growth Inhibition Assay | 0-50 μM | IC50=16 μM | 25750273 | ||
| TR-LN229 | Growth Inhibition Assay | 0-50 μM | IC50=77 μM | 25750273 | ||
| U87 | Apoptosis Assay | 0–200 µM | 24 h | enhances CQ induced apoptosis | 25681668 | |
| U251MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U118 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U87 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U118 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U87 | Growth Inhibition Assay | 250/350 μM | 48 h | increases the percentage of cells in S and G2/M cotreated with TMX | 25554223 | |
| A375 | Growth Inhibition Assay | 48 h | IC50=265 μM | 25524552 | ||
| A2058 | Growth Inhibition Assay | 48 h | IC50=12 μM | 25524552 | ||
| M238 | Growth Inhibition Assay | 48 h | IC50=40 μM | 25524552 | ||
| M249 | Growth Inhibition Assay | 48 h | IC50=254 μM | 25524552 | ||
| M21 | Growth Inhibition Assay | 48 h | IC50=221 μM | 25524552 | ||
| U251 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| LN229 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| U373MG-LUC | Growth Inhibition Assay | 72 h | IC50>600 μM | 25431953 | ||
| U87 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U373 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U343 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87MG-luc2 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| U87 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| U251 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| A172 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| SNB19V | Growth Inhibition Assay | 7 d | DMSO | GI50=35.7±12 μM | 25277441 | |
| SNB19M | Growth Inhibition Assay | 7 d | DMSO | GI50=469.9±88 μM | 25277441 | |
| SNB19VR | Growth Inhibition Assay | 7 d | DMSO | GI50=280.2±18 μM | 25277441 | |
| U373V | Growth Inhibition Assay | 7 d | DMSO | GI50=68.0±32 μM | 25277441 | |
| U373M | Growth Inhibition Assay | 7 d | DMSO | GI50=368.7±86 μM | 25277441 | |
| U373VR | Growth Inhibition Assay | 7 d | DMSO | GI50=288.8±33 μM | 25277441 | |
| U87MG | Growth Inhibition Assay | 7 d | DMSO | GI50=38.3±20 μM | 25277441 | |
| HCT116 | Growth Inhibition Assay | 7 d | DMSO | GI50=579.9±32 μM | 25277441 | |
| DLD1 | Growth Inhibition Assay | 7 d | DMSO | GI50=501.4±93 μM | 25277441 | |
| MRC5 | Growth Inhibition Assay | 7 d | DMSO | GI50=449.4±8 μM | 25277441 | |
| SNB19V | Function Assay | 100 μM TMZ | 0-72 h | increases γH2AX expression between 16 and 72 h | 25277441 | |
| T98G | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| U251 | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| T98G | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U251 | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U-87 MG | Growth Inhibition Assay | 72 h | IC50=0.93 mM | 25245332 | ||
| U-118 MG | Growth Inhibition Assay | 72 h | IC50=1.05 mM | 25245332 | ||
| U87 | Growth Inhibition Assay | 24 h | IC50=260.34 μM | 25173233 | ||
| U87 GSLCs | Growth Inhibition Assay | 24 h | IC50=766.11 μM | 25173233 | ||
| U87MG | Growth Inhibition Assay | 72 h | IC50=15.625 μM | 25050915 | ||
| U251 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| U87 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231-br | Growth Inhibition Assay | 0-10 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| HCC-1937 | Growth Inhibition Assay | 0-300 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231 | Growth Inhibition Assay | 0-40 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-468 | Growth Inhibition Assay | 0-500 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| T47D | Growth Inhibition Assay | 0-100 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MCF7 | Growth Inhibition Assay | 0-1000 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| Hs683 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=128.9 μM | 24495907 | |
| U87 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=18.45 μM | 24495907 | |
| LNZ308 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=326.7 μM | 24495907 | |
| U87 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Growth Inhibition Assay | 24 h | IC50=86.29 ± 1.58 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 48 h | IC50=75.34 ± 1.02 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 72 h | IC50=72.42 ± 1.45 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 96 h | IC50=69.82 ± 3.04 μM | 24326954 | ||
| T98G | Growth Inhibition Assay | 0-750 μM | 72/96 h | inhibits cell viability in a dose dependent manner | 24324080 | |
| U251-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| D54-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| SHG-44 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=9.73 ± 2.12 μM | 24065569 | |
| U373 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=10.13 ± 1.02 μM | 24065569 | |
| HT-29 | Function Assay | 500 μM | 24/48 h | enhances the levels of γ-H2AX | 24038068 | |
| PC-3 | Growth Inhibition Assay | 0-25 μM | 48 h | inhibits cell growth which can be potentiated by lycopene | 23746934 | |
| PC-3 | Apoptosis Assay | 25 μM | 48 h | induces apoptosis which can be potentiated by lycopene | 23746934 | |
| T98G | Growth Inhibition Assay | 50-400 μM | 144 h | inhibits cell viability in a dose dependent manner | 23715499 | |
| U87-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| U251-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| LNT-229 | Growth Inhibition Assay | 3-100 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| T98G | Growth Inhibition Assay | 10-700 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| U87 | Function Assay | 100 µM | 3 h | elevates the levels of pChk1 and pChk2 | 23667469 | |
| HCT116 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| HCT3-6 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| U-87 | Growth Inhibition Assay | 0-40 μM | 12 d | inhibits cell growth in a dose-dependent manner | 23645729 | |
| U-87 | Apoptosis Assay | 0-40 μM | 3/6 d | induces apoptosis in both dose- and time-dependent manner | 23645729 | |
| U-87 | Function Assay | 0-40 μM | 3/6 d | induces autophagy in both dose- and time-dependent manner | 23645729 | |
| GB-SCC010 | Growth Inhibition Assay | 4 d | IC50=226 μM | 23612755 | ||
| GB-SCC026 | Growth Inhibition Assay | 4 d | IC50=53.1 μM | 23612755 | ||
| GB-SCC028 | Growth Inhibition Assay | 4 d | IC50=167 μM | 23612755 | ||
| U87 | Growth Inhibition Assay | 4 d | IC50=45.2 μM | 23612755 | ||
| U87 stem cell | Growth Inhibition Assay | 4 d | IC50=66.7 μM | 23612755 | ||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 7739008 | |||
| GM892 A | Cytotoxicity assay | IC50 = 7.6 μM | 7739008 | |||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 12459014 | |||
| HCT116 | Cytotoxicity assay | 4 days | IC50 = 4.34 μM | 19800803 | ||
| SNB75 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SNB75 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| C6 | Antiproliferative assay | 100 uM | 48 hrs | Antiproliferative activity against rat C6 cells at 100 uM after 48 hrs by neutral red incorporation assay | 22268526 | |
| SF295 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SF295 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| U87 | Antiproliferative assay | 5 days | IC50 = 49 μM | 22608389 | ||
| U138MG | Cytotoxicity assay | 48 hrs | IC50 = 26 μM | 23069682 | ||
| C6 | Cytotoxicity assay | 48 hrs | IC50 = 34 μM | 23069682 | ||
| A2780 | Antitumor assay | 5 days | IC50 = 8.5 μM | 23895620 | ||
| A2058 | Antitumor assay | 5 days | IC50 = 35.5 μM | 23895620 | ||
| SNB19 | Antitumor assay | 5 days | IC50 = 37 μM | 23895620 | ||
| M8 | Apoptosis assay | 50 to 100 uM | 48 hrs | Induction of apoptosis in human M8 cells assessed as apoptotic/necrotic cells at 50 to 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| A2780 | Cytotoxicity assay | 5 days | Cytotoxicity against human A2780 cells after 5 days by MTT assay | 24900418 | ||
| A2780/CP70 | Cytotoxicity assay | 5 days | Cytotoxicity against MMR-deficient human A2780/CP70 cells after 5 days by MTT assay | 24900418 | ||
| GBM 047T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 047T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| GBM 464T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 464T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| U87MG | Function assay | 3 hrs | Induction of DNA alkylation in human U87MG cells assessed as increase in N7-MedG formation after 3 hrs by LC-MS/MS analysis | 27614414 | ||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as induction of slow tumor growth at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as increase in mouse survival at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under normoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under hypoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| C6 | Cytotoxicity assay | 4 days | EC50 = 16.5 μM | ChEMBL | ||
| U87 | Cytotoxicity assay | 72 hrs | IC50 = 19.38 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 35.7 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 45.6 μM | ChEMBL | ||
| U373 | Function assay | 100 uM | 72 hrs | Induction of double stranded DNA break in empty vector transfected human U373 cells assessed as increase in gamma-H2AX level at 100 uM after 72 hrs by flow cytometry | ChEMBL | |
| Glioma | Antitumor assay | Antitumor activity against Homo sapiens (human) Glioma cells xenografted in transgenic mouse assessed as mouse survival | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | TMZ(Temozolomide) is a monofunctional SN-1 alkylating agent that can modify nitrogen atoms in the DNA ring and the extracyclic oxygen group, chemically converted to MTIC and degrades to methyldiazonium cation, which transfers methyl groups to DNA at physiologic pH. A DNA damage inducer in L-1210 and L-1210/BCNU cells. Temozolomide induces apoptosis and exhibits antitumor activity. | |
|---|---|---|
| Features | Methazolastone is a second-generation alkylating agent. | |
| Targets |
|
| In vitro | ||||
| In vitro | Methazolastone causes formation of DNA alkali-labile sites which are present in similar amounts and repaired at a similar rate in L-1210 and L-1210/BCNU cell lines. In L-1210 but not in L-1210/BCNU methazolastone induces an arrest of cells in SL-G2-M phases.[1] Methazolastone sensitivity of both chemo-sensitive and resistant cells (D54-R and U87-R) is enhanced significantly under hyperoxia. Both Methazolastone and hyperoxia are associated with increased phosphorylation of ERK p44/42 MAPK (Erk1/2), but to a lesser extent in D54-R cells, suggesting that Erk1/2 activity may be involved in regulation of hyperoxia and Methazolastone-mediated cell death. Hyperoxia enhances Methazolastone toxicity in GBM cells by induction of apoptosis, possibly via MAPK-related pathways. [2] Methazolastone induces in monocytes the DNA damage response pathways ATM-Chk2 and ATR-Chk1 resulting in p53 activation. [3] Chronic Methazolastone exposure results in acquired Methazolastone-resistance and elevates miR-21 expression. [4] Methazolastone treatment triggers endoplasmic reticula (ER) stress with increased expression of GADD153 and GRP78 proteins, and deceases pro-caspase 12 protein. Methazolastone induces autophagy through mitochondrial damage- and ER stress-dependent mechanisms to protect glioma cells. [5] |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | L-1210 and L-1210/BCNU cells | ||
| Concentrations | 0 μM -100 μM | |||
| Incubation Time | l hours | |||
| Method | L-1210 and L-1210/BCNU cells are seeded at 0.2 × 104 cells/mL and incubated for 24 hours. The cultures are treated with Methazolastone for l hours at 37oC, then washed twice in PBS by centrifugation and resuspended in fresh medium. Controls and treated samples are diluted in fresh medium 1:4 at 48 hours and 1:2 at 96 hours. Using these dilutions cell concentrations throughout the experiments are between 3 × 105 and 8 × 105/mL. Control growth is logarithmic in this range. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | DR5 / c-FLIP / Survivin / XIAP pERK / ERK / p-p38 / p38 |
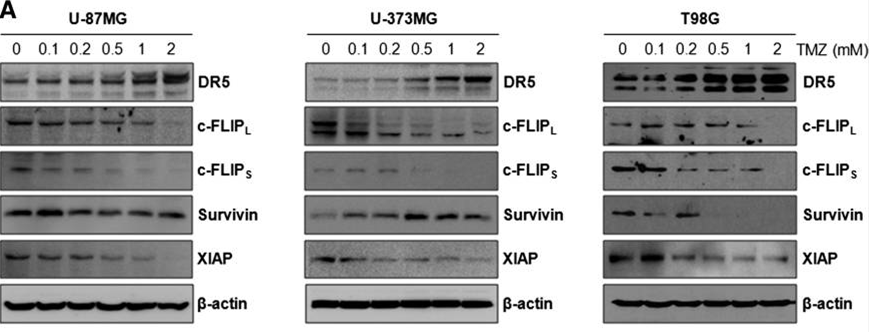
|
24436439 | |
| Growth inhibition assay | Cell viability |
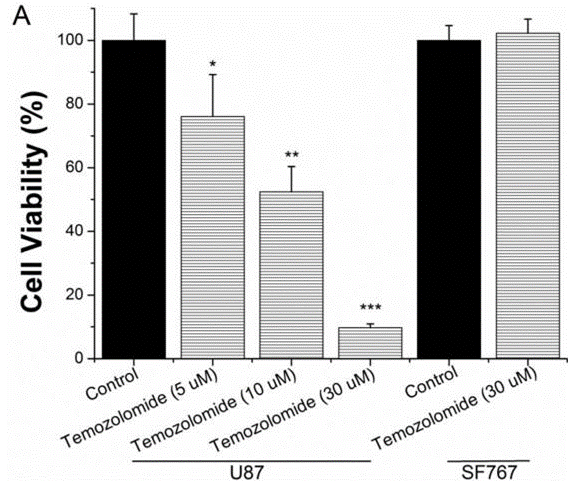
|
25751281 | |
| Immunofluorescence | Phalloidin / Phospho-H2A.X cleaved caspase-3 |
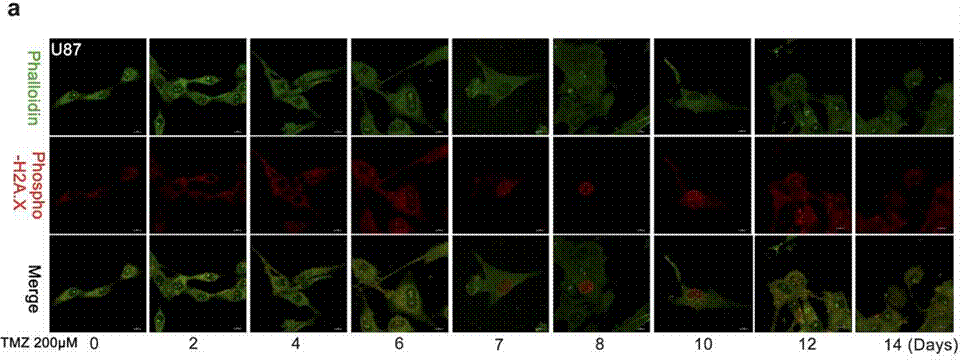
|
27375225 | |
| In Vivo | ||
| In vivo |
After a daily i.p. dose of 40 mg/kg for 5 consecutive days (days 1-5 after tumor transplant), methazolastone increases life-span by 86% in L-1210 and 22% in L-1210/BCNU. In L-1210/BCNU no effect is seen after 100 μM or 200 μM treatment; only 400 μM methazolastone produced an accumulation of cells in premitotic phase but much less than in L-1210. In L-1210/BCNU the maximum accumulation of cells in SL-G2-M is, after 48 hours-72 hours, approximately 30% as compared to 23% in untreated cells. Cells accumulates in SL-G2-M occurred too when L- 1210 leukemia-bearing mice are treated i.v. with methazola stone (40 mg/kg). No such effect is seen on L-1210/BCNU cells from mice given the same drug dose. [1] |
|
|---|---|---|
| Animal Research | Animal Models | DBA/2 mice with L-1210 and L-1210/BCNU cells |
| Dosages | 40 mg/kg | |
| Administration | Administered via i.v. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06161974 | Not yet recruiting | High Grade Glioma|Astrocytoma|Astrocytoma Grade III|Astrocytoma Grade IV|Diffuse Intrinsic Pontine Glioma|WHO Grade III Glioma|WHO Grade IV Glioma|Metastatic Brain Tumor|Diffuse Midline Glioma H3 K27M-Mutant|Thalamus Tumor|Spinal Tumor|IDH1 Mutation|IDH1 R132|IDH1 R132C|IDH1 R132H|IDH1 R132S|IDH1 R132G|IDH1 R132L|Oligodendroglioma | Rigel Pharmaceuticals|Nationwide Children''s Hospital | June 15 2024 | Phase 2 |
| NCT04967690 | Not yet recruiting | Newly Diagnosed Glioblastoma | Double Bond Pharmaceutical AB | January 2024 | Phase 1 |
| NCT05128734 | Not yet recruiting | Breast Cancer Triple Negative | AHS Cancer Control Alberta | December 1 2023 | Phase 2 |
| NCT05698524 | Recruiting | Recurrent High Grade Glioma|Anaplastic Astrocytoma|Anaplastic Oligodendroglioma|Glioblastoma|Gliosarcoma | University of Nebraska|Xynomic Pharmaceuticals Inc. | June 26 2023 | Phase 1 |
| NCT04945148 | Not yet recruiting | Glioblastoma IDH-wildtype | Hopital Foch|National Cancer Institute France | May 2023 | Phase 2 |
| NCT05885386 | Recruiting | Pheochromocytoma|Paraganglioma | Peking Union Medical College Hospital | April 1 2023 | Phase 2 |
Chemical lnformation & Solubility
| Molecular Weight | 194.15 | Formula | C6H6N6O2 |
| CAS No. | 85622-93-1 | SDF | Download TMZ(Temozolomide) SDF |
| Smiles | CN1C(=O)N2C=NC(=C2N=N1)C(=O)N | ||
| Storage (From the date of receipt) | 3 years-20°C (in the dark)powder | ||
|
In vitro |
DMSO : 39 mg/mL ( (200.87 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : 7 mg/mL Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy TMZ(Temozolomide) | TMZ(Temozolomide) ic50 | TMZ(Temozolomide) price | TMZ(Temozolomide) cost | TMZ(Temozolomide) solubility dmso | TMZ(Temozolomide) purchase | TMZ(Temozolomide) manufacturer | TMZ(Temozolomide) research buy | TMZ(Temozolomide) order | TMZ(Temozolomide) mouse | TMZ(Temozolomide) chemical structure | TMZ(Temozolomide) mw | TMZ(Temozolomide) molecular weight | TMZ(Temozolomide) datasheet | TMZ(Temozolomide) supplier | TMZ(Temozolomide) in vitro | TMZ(Temozolomide) cell line | TMZ(Temozolomide) concentration | TMZ(Temozolomide) nmr







































